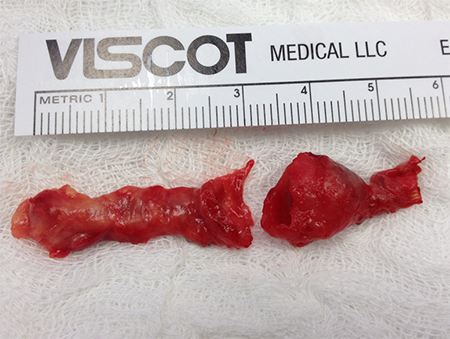
Figure 7. Histopathology of the radial artery aneurysm, indicating a cluster of coccus shaped bacteria.
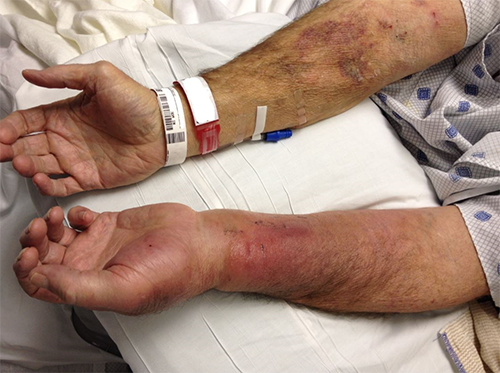
Four days postoperatively, the patient’s vitals had normalized and white blood count had normalized to 6,300 cells/μL. The nafcillin infusion was replaced with oral cephalexin for six weeks following discharge. Final debridement and delayed primary closure occurred on postoperative day twelve. At one month postclosure, the patient’s wound had completely healed. At six-month follow-up, he had completely recovered without long-term sequelae.
Discussion
This case is particularly unique in that it is represents the first report of a true mycotic aneurysm as a complication of percutaneous cardiac catheterization utilizing radial artery access. The diagnosis of true aneurysm is evident in this case, as there was marked dilation of the arterial wall including all three layers (adventitia, media, and intima) without evidence of homogenous dissection of the arterial wall. Additionally, evidence of bacteria in the aneurysmal arterial wall and surrounding tissue define it as a mycotic aneurysm. Although the etymology of mycotic aneurysms refer to that of fungi, and more specifically fungating vegetations associated with bacterial endocarditis as noted by Dr. William Osler in 1885, it has become common place to use the term mycotic to describe aneurysms stemming from other bacterial sources as well.5
Mycotic aneurysms of the upper extremities are rare (only 10% of all cases). Arterial trauma from intravenous (IV) drug abuse and interventional procedures are the most common causes of mycotic aneurysm in the upper extremities, with the brachial artery being by far the most common site.4 Our patient’s radial artery injury was obviously caused by the catheterization process as he was not an IV drug abuser. Our patient responded appropriately to therapy and his source of sepsis was clear; therefore, further work-up, such as a transesophageal echocardiogram, was not indicated.
The key to diagnosis in this case was the patient’s systemic toxicity and high fever which distinguish it from commonly seen sterile inflammation. Sterile inflammation is a consequence of damage associated molecular pattern molecules (DAMPs) responding to chemical, physical, or metabolic stimuli in the absence of responsible microorganisms.6 It is a common finding in percutaneous coronary intervention and occurs in 1–2 percent of cases where hydrophilic-coated sheaths are used. Akin to a foreign body reaction, it is usually treated with anti-inflammatory measures.7 Cultures from these patients are negative and antibiotics are not indicated. Patients suffering from sterile inflammation generally present two to three weeks post procedure and without intervention the inflammation will resolve over several months.7
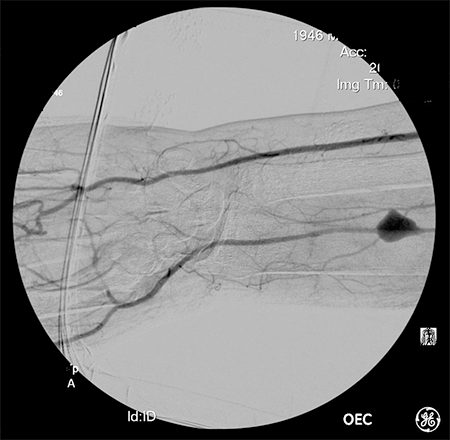
Timely surgical debridement with wound management and IV antibiotics was imperative in this case; however, it was important to define the arterial anatomy prior to debridement and aneurysm resection. Had the palmar arch been incomplete, consideration would have been given for autogenous vein bypass grafting for hand revascularization. Given the results of the modified Allen’s test and subsequent arteriography, we were confident that resection of the radial artery would not lead to hand necrosis.
Conclusion
Although this is the first true mycotic aneurysm of the radial artery we have seen, it will likely become more common due to the steadily increasing number of cardiac catheterizations utilizing radial artery access. Despite meticulous sterile techniques and chlorhexidine skin preparation, these complications still occur. Mycotic aneurysm should be part of the differential diagnosis when confronted with progressively worsening access site inflammation and pain that does not respond to empiric treatment of sterile inflammation.
Lessons Learned
True mycotic aneurysm may appear similar to common sterile inflammation following radial artery access procedures. Although this is the first reported case, we expect radial artery mycotic aneurysm to become more frequent as radial access becomes commonplace. Timely surgical intervention and antibiotic treatment contributed to quick resolution in this case.
Authors
Adams Va, Vernon Sb, Battin Db, Hughes TGa
Correspondence Author
Seth A. Vernon, MD
Mowery Clinic
737 East Crawford Street
Salina, KS 67401
785-827-7261
svernon@moweryclinic.com
Author Affiliations
- University of Kansas School of Medicine—Salina
400 South Santa Fe Avenue
Salina, KS 67401
- Mowery Clinic
737 East Crawford Street
Salina, KS 67401
References
- Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. The Lancet. 2011;377(9775):1409-20. DOI: 10.1016/S0140-6736(11)60404-2
- Amin AP, House JA, Safley DM, et al. Costs of transradial percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6:827-834. DOI: 10.1016/j.jcin.2013.04.014
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999 Sep;138(3 Pt 1):430-6. PMID: 10467191
- Leon LR, Psalms SB, Labropoulos N, Mills JL. Infected upper extremity aneurysms: a review. Eur J Vasc Endovasc Surg. 2008 Mar;35(3):320-31. DOI: 10.1016/j.ejvs.2007.10.014
- Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J. 1885;1(1262):467-470. PMID: 20751186
- Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826-837. DOI: 10.1038/nri2873
- Kozak M, Adams DR, Ioffreda MD, et al. Sterile inflammation associated with transradial catheterization and hydrophilic sheaths. Catheter Cardiovasc Interv. 2003;59(2):207-13. DOI: 10.1002/ccd.10522