Figure 2. Hematoxylin and eosin stained section at 400x magnitude showing a richly vascular tumor comprised of fairly uniform alveolar groups of neoplastic cells (“zellballen”) characteristic of paraganglioma. Consistent with paraganglioma, this section also stained strongly positive for neuroendocrine markers chromogranin A and synaptophysin; immunostain for S100 protein was also positive. Stains for pancytokeratin and AE1/AE3 melan A were negative. Focally, the tumor demonstrated extension through the thin capsule without obvious mitotic figures or tumor necrosis.
Discussion
Paraganglia are small, extra-adrenal organs comprised primarily of chromaffin cells—specialized neuroendocrine cells of embryonic neural crest origin—and are classified as either sympathetic or parasympathetic based on their anatomic distribution. Sympathetic paraganglia are found symmetrically along the paravertebral axis, from the superior cervical ganglion to the bladder, whereas parasympathetic ganglia are localized to the skull base and neck.1
Paragangliomas are richly vascular, encapsulated neuroendocrine tumors that can develop from paraganglia wherever they are distributed, most commonly in the head and neck. They are closely related to pheochromocytomas, chromaffin cell tumors of the adrenal gland, and represent 10 percent to 18 percent of all chromaffin-tissue tumors.1 Paragangliomas are rare—their estimated incidence is 1 in 300,000 and are equally distributed among men and women.2 Depending on their biochemical activity, paragangliomas are categorized as either functional or non-functional. Functional paragangliomas comprise 30 to 60 percent of the tumors and are so-named because they produce and secrete catecholamines, which cause paroxysms of hypertension, palpitations, tremors, headache and diaphoresis.3 Non-functional paragangliomas lack this activity and are physiologically silent. They usually come to physician attention through genetic screening, complaints related to mass effect on head and neck structures or incidental discovery on CT or MRI. In one series, only 20 percent of patients with benign paragangliomas had documented catecholamine hypersecretion; the majority presented secondary to mass effect or incidental imaging findings.1,4 Although most paragangliomas are benign, surgical resection is recommended to prevent metastasis, which occurs in 20 to 42 percent of cases.3 Radiation is a viable alternative for poor surgical candidates.1,4
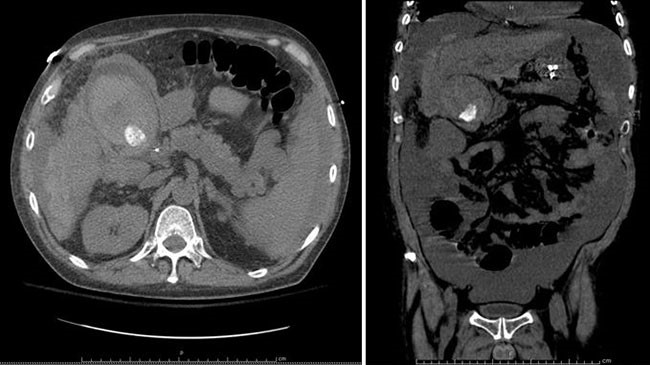
As illustrated by our case, preoperative planning and surgical management are challenging in asymptomatic patients with incidental abdominal masses. Despite CT imaging, which is 90 percent sensitive for paragangliomas and the modality of choice for localization, our patient’s tumor was undetectable.3 PET imaging also failed to elucidate the correct preoperative diagnosis, and accordingly, the mass was treated as a metastasis. The diagnosis became clear only after complete pathologic evaluation. In fact, 27 percent of patients with benign, incidental thoracic or abdominal paragangliomas require pathologic examination of resected specimens for definitive diagnosis as preoperative imaging and gross intraoperative inspection are insufficient.4 Patients who undergo resection of undiagnosed paragangliomas are at increased risk for morbidity and mortality because surgical manipulation of even non-functional tumors can cause transient release of catecholamines and hemodynamic instability. Thus, whenever a paraganglioma is suspected, patients should receive alpha- and beta-adrenergic blockade as protection from intraoperative catecholamine hypersecretion.
To the best of our knowledge, this is the first case of colon adenocarcinoma and a sporadic, non-functional paraganglioma presenting within one year of each other. The coincidence of paragangliomas and solid organ tumors is not itself uncommon because 10 to 50 percent of paragangliomas are hereditary, manifesting alongside other neoplasms in autosomal dominant diseases such as neurofibromatosis type 1, von Hippel-Lindau disease, Carney triad, and multiple endocrine neoplasia type 2.1 However patients with these syndromes typically present with functional paragangliomas, and colon adenocarcinoma is rarely involved. Given our patient’s presentation and lack of family history, her tumors were deemed unrelated and genetic screening was deferred.
Conclusion
PET-CT imaging identified a hypermetabolic mass in the superior abdomen of a patient nine months after her right hemicolectomy for colon adenocarcinoma. Given her lack of symptoms and the suspicion that this lesion was related to her colon cancer, the patient underwent an exploratory laparotomy less than three months later with enucleation of what turned out to be a non-functional, retroperitoneal paraganglioma. This unique case highlights both the challenges involved in the diagnosis of asymptomatic chromaffin-tissue tumors and the importance of radiologic surveillance in patients with colorectal cancer.
Lessons Learned
Asymptomatic paragangliomas can masquerade as metastases or local recurrence in patients with previously resected malignancies. This case highlights the importance of surveillance imaging in these patients as well as the challenge in identifying non-functional paragangliomas preoperatively.
Authors
Leah Winer, MD; Sarah M. Kling, MD; Anthony J. Prestipino, MD; James P. Casey, MD; Pankhuri Jha, BS; Ernest L. Rosato, MD, FACS; and Scott D. Goldstein, MD, FACS, ASCRS
Correspondence Author
Dr. Scott D. Goldstein
Department of Surgery
Division of Colon and Rectal Surgery
Thomas Jefferson University
1100 Walnut Street
Suite 500
Philadelphia, PA 19107
215-570-1464
scott.goldstein@jefferson.edu
Author Affiliations
Thomas Jefferson University
Sidney Kimmel Medical College
Philadelphia, PA 19107
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Young WF. Paragangliomas: Clinical Overview. Ann N Y Acad Sci. 2006;1073:21-29.
- McNicol AM. Histopathology and immunohistochemistry of adrenal medullary tumors and paragangliomas. Endocrinol Pathol. 2006;17(4):329-336.
- Sangster G, Do D, Previgliano C, et al. Primary Retroperitoneal Paraganglioma Simulating a Pancreatic Mass: A Case Report and Review of the Literature. HPB Surg. 2010;2010:645728.
- Erickson D, Kudva YC, Ebersold MJ, Thompson GB, van Heerden JA, Young WF Jr. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001;86(11):5210-5216.