Figure 3. Ancillary testing. A—C: Immunohistochemistry, peroxidase. Immunohistochemical stain reveals that the capillary-like vascular spaces are positive for CD31 (A), CD34 (B) and CD8 (C), a profile consistent with splenic vasculature. (D): In-situ hybridization (ISH). No Epstein-Barr virus-positive cells were identified via EBER-ISH. (E—F) Immunohistochemistry, peroxidase. A significant number of IgG positive plasma cells was seen (E), but only rare IgG4 positive cells were identified (F) estimated to represent less than five percent of the total IgG positive plasma cells.
Vascular structures were positive for both CD31 and CD34 and negative for CD8. Of note, no evidence of Epstein-Barr virus (EBV) infection was seen on EBV-encoded ribonucleic acid (EBER) in-situ hybridization, and no increased IgG4+ plasma cells were identified on IgG and IgG4 immunohistochemistry. Collectively, these findings are compatible with the diagnosis of SANT.
At her four-week follow-up appointment, the patient denied ongoing symptoms of pain. She reported occasional nausea for which resolved after an additional four weeks.
Discussion
SANT is a benign, nodular, vascular proliferation of splenic red pulp that is often associated with sclerosis. Its exact etiology is not fully understood; however, one theory is that SANT is the end-stage of a variety of benign splenic conditions, including inflammatory pseudotumor, hamartoma, and hematoma.2 Although SANT was originally cited to occur more often in females, this preponderance has declined as more reports are published.3
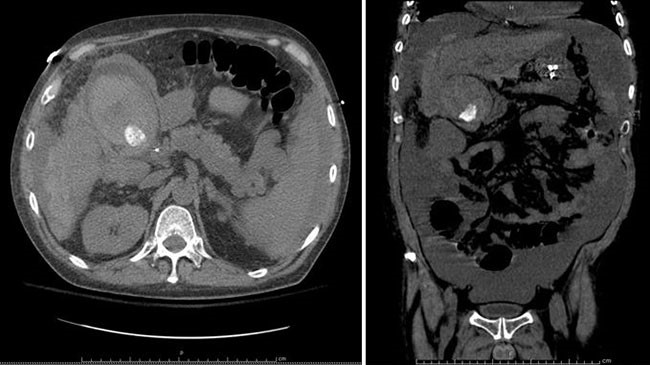
The radiographic features of SANT have been previously described on CT and MRI, which closely correlates with our findings.4,5 On CT, the lesion appears as a hypodense mass with heterogeneous enhancement. It has a well-demarcated border and nodular peripheral enhancement suggestive of a hypervascular rim and is without calcifications. On T1WI contrast-enhancing MRI, the lesion shows centripetal and progressive enhancement without signs of cystic changes or necrosis. These radiologic findings may be suggestive of benign vascular lesions such as hemangiomas; however, they can also be malignant hemangiomas such as angiosarcomas. Therefore, it is important to definitively characterize these lesions by histopathology.6
Most reported cases of SANT are diagnosed after splenectomy following an incidental radiographic finding; however, there is one report of diagnosis by ultrasound-guided percutaneous core needle biopsy.7 To date, there have been no reported asymptomatic cases or instances where SANT has been observed without surgical intervention. This may be, in part, due to the requirement of histology for the definitive diagnosis.
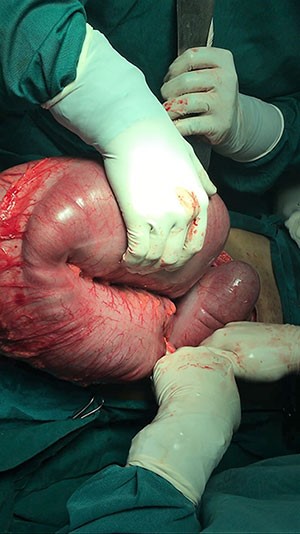
On gross examination, SANT is usually a solitary, well-circumscribed nodule that is distinct from the surrounding splenic parenchyma. Histologically, it has a vaguely lobular architecture surrounded by a hyaline shell, with markedly cellular blood vessels similar to those seen in hemangiomas.8 Its stroma consists of myxoid and sclerotic fibrous tissue with some myofibroblasts, lymphoplastmacytic cells, and hemosiderin-containing macrophages. Immunohistochemical analysis has suggested SANT to be a predominantly polyclonal reactive lesion, lacking evidence of any relationship with IgG4-related sclerosing lesions, or EBV-positive stromal cells.9 SANT has traditionally demonstrated three distinct patterns of immune staining: CD34+/CD31+/CD8-, CD34-/CD31+/CD8+ , and CD34-/CD31+/CD8-, which indicates derivation from capillaries, splenic sinusoidal lining cells, or small veins, respectively.10 Early lesions have also demonstrated perivascular concentric fibrosis, as was seen in this case.6
Splenectomy is the treatment of choice for SANT, and no instance of recurrence has been reported. It is important, however, to note that the initial presentation can be somewhat ambiguous. In our case, we opted for a laparoscopic splenectomy to minimize length of stay and to shorten the postoperative recovery time. Morcellation to extract the spleen was avoided due to the uncertain diagnosis of the lesion and to prevent inadvertent spread of a potential malignancy. We extracted the intact spleen in a large laparoscopic bag by extending the umbilical port. This may be of special pertinence given that there have been a few reported instances of multifocal lesions involving anywhere from two to eight nodules, as these presentations may further enhance a clinician's suspicion of a malignant etiology.3
Lastly, it is important to ensure proper vaccination to prevent overwhelming postsplenectomy sepsis secondary to encapsulated bacteria. The timing of vaccinations has been the topic of a longstanding debate, and there is no Class I data identifying the appropriate timing for presplenectomy. It is generally accepted that patients undergoing elective splenectomy should be vaccinated two weeks prior to the procedure; this guideline is supported by Class III data.11 Those undergoing emergent or urgent splenectomy should receive vaccinations prior to discharge.
Conclusion
SANT is a rare splenic lesion and cause of left upper quadrant pain. The surgical approach should consider the possibility of malignancy, and morcellation of the spleen should be avoided. No recurrent lesions have been reported after surgical removal.
Lessons Learned
Sclerosing angiomatoid nodular transformation of the spleen is a rare, benign vascular growth the can present with vague symptoms. Imaging suggests vascular involvement, but diagnosis requires histologic evaluation. Here we report a case managed by laparoscopic splenectomy with port site extension for intact removal of the spleen.
Authors
Andre Critsinelis, BS
Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA
Nahir Cortes-Santiago, MD, PhD
Department of Pathology and Immunology, Baylor College of Medicine, Houston, Texas, USA
Vasantha L. Gali, MD
Department of Pathology and Immunology, Baylor College of Medicine, Houston, Texas, USA
Christie Finch, MD
Department of Pathology and Immunology, Baylor College of Medicine, Houston, Texas, USA
Stephanie D. Gordy, MD
Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA
Correspondence
Dr. Stephanie Gordy
Baylor College of Medicine
1 Baylor Plaza, BCM390
Houston, TX 77030
Email: Stephanie.Gordy@bcm.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): Report of 25 cases of a distinct benign splenic lesion. Am J Surg Pathol. 2004;28:1268-1279.
- Falk GA, Nooli NP, Morris-Stiff G. Sclerosing angiomatoid nodular transformation (SANT) of the spleen: Case report and review of the literature. Int J Surg Case Rep. 2012;3:492-500.
- Cao Z, Wang Q, Li J, Xu J, Li J. Multifocal sclerosing angiomatoid nodular transformation of the spleen: a case report and review of literature. Diagnostic Path. 2015;10.
- Thacker C, Korn R, Millstine J, Harvin H, Van Lier Ribbink JA, Gotway MB. Sclerosing angiomatoid nodular transformation of the spleen: CT, MR, PET, and 99mTc-sulfure colloid SPECT CT findings with gross and histopathological correlation. Abdominal Imaging. 2010;35:683-689.
- Kim HJ, Kim KW, Yu ES, Byun JH, Lee SS, Kim JH, Lee JS. Sclerosing angiomatoid nodular transformation of the spleen: Clinical and radiologic characteristics. Acta Radiologica. 2012;53:701-706.
- Pradhan D, Mohanty SK. Sclerosing Angiomatoid Nodular Transformation of the Spleen. Arch Pathol Lab Med. 2013;137:1309-1312.
- Gutzeit A, Stuckmann G, Dommann-Scherrer C. Sclerosing angiomatoid nodular transformation (SANT) of the spleen: sonographic finding. J Clin Ultrasound. 2009;37:308-311.
- Gomez-Rubio M, Lopez-Cano A, Rendon P, Munoz-Benvenuty A, Macias M, Garre C, Segura-Cabral JM. Safety and Diagnostic Accuracy of Percutaneous Ultrasound-Guided Biopsy of the Spleen: A Multicenter Study. J Clin Ultrasound. 2009;37:445-450.
- Chang KC, Lee JC, Wang YC, Hung LY, Huang Y, Huang WT, Wang RC, Chen TS, Tasi YS, Medeiros LJ. Polyclonality in sclerosing angiomatoid nodular transformation of the spleen. Am J Surg Pathol. 2016;10:1343-1351.
- Agrawal M, Shantveer G, Srinivas BH, Megha S, Bheerappa N, Challa S. Sclerosing Angiomatoid Nodular Transformation of the Spleen: A New Entity or a New Name? Turk J Pathol. 2013;32:205-210.
- Post-splenectomy Vaccine Prophylaxis. Orlando Regional Medical Center Department of Surgical Education. http://www.surgicalcriticalcare.net/Guidelines/splenectomy_vaccines.pdf [Accessed 03/28/2018].