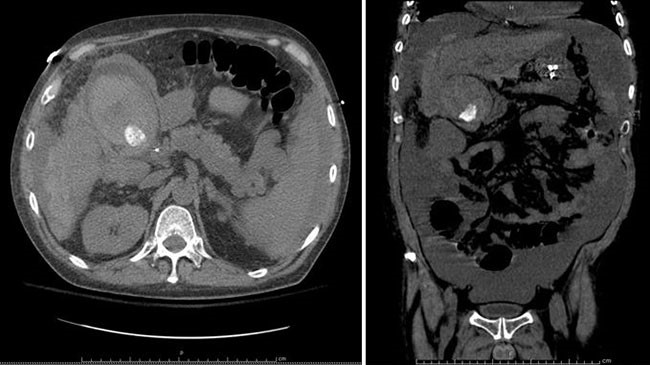
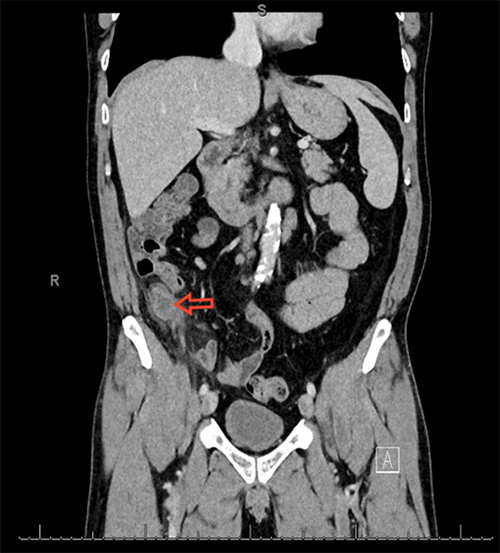
Figure 2. CT Scan of Abdomen/Pelvis demonstrating a mass in the gastric remnant. Note proximity of the gastric pouch staple line to the remnant stomach.
The patient underwent esophagogastroduodenoscopy (EGD) which additionally showed a small nonbleeding marginal ulcer at the gastrojejunostomy. A double balloon-assisted enteroscopy was not performed at this admission, as this hospital did not have the capabilities to perform this procedure. The patient was discharged after a five-day hospital admission on an oral PPI and iron supplementation and with instructions to avoid NSAIDs; the final diagnosis was NSAID-induced gastric and marginal ulcers.
One week later, the patient re-presented to our hospital with recurrent melena, anemia, shortness of breath, and epigastric pain. Her hemoglobin was 8.7g/dL, and she was hemodynamically normal. The patient was re-initiated on intravenous PPI therapy and sucralfate. EGD was repeated and was notable for evidence of a Roux-en-Y gastrojejunostomy with healthy-appearing mucosa with no bleeding or ulcers. A Helicobacter pylori serum antigen was sent and was negative. This time, the patient did undergo a double balloon-assisted enteroscopy, which was notable for a healthy-appearing mucosa of the gastrojejunostomy and enteroenterostomy, a roux limb of approximately 300 cm, and intubation of the biliopancreatic limb confirmed dark black-brown liquid contents consistent with liquefied hematoma. The proximal duodenum and remnant stomach were unable to be accessed due to sharp angulations and looping. The etiology of this UGIB was again presumed to be NSAID-induced ulceration of the excluded stomach or proximal duodenum.
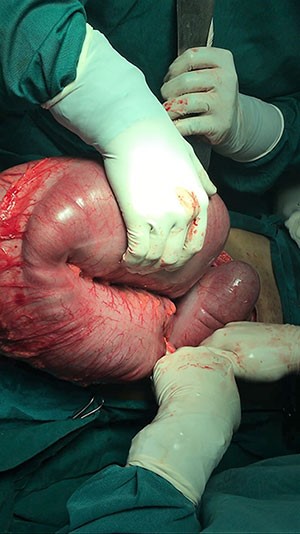
Over the subsequent 24 hours, the patient's hemoglobin decreased by two grams, and she became hypotensive. Given her persistent bleeding and hemodynamic instability despite maximal medical therapy, she was taken to the operating room for diagnostic laparoscopy, laparoscopic-assisted gastroduodenoscopy, as previously described in the literature,2-5 and possible remnant gastrectomy. This required an extensive adhesiolysis, given her multiple prior foregut and abdominal surgeries. The gastric remnant was identified and was distended. A 15 mm trocar was placed into the gastric remnant with endoscopic visualization of a large volume of clot and dark blood. A second trocar was then placed into the gastric remnant, and a large suction catheter was utilized to aspirate and clear the blood and clot under direct visualization. Endoscopy was notable for a 3 cm ulcer in the distal antrum on the lesser curvature with surrounding inflammation. The endoscope was advanced into the duodenum through a narrowed prepyloric channel, with visualization of clear bile. The duodenum was examined with no evidence of ulceration. Given these findings, we elected to perform a near-total gastrectomy with resection of the gastric remnant. This was performed with difficulty, as the gastric remnant in the prepyloric region was densely adherent to the lateral aspect of the gastric pouch and to the liver (Figure 2). Final pathology revealed high-grade invasive gastric adenocarcinoma, stage IIIA, pT4aN1M0, with lymphovascular invasion, perineural invasion, and two of three involved lymph nodes with satellite nodules into the peri-gastric soft tissues in a background of diffuse and chronic gastritis.
Discussion
The incidence of bariatric surgery is increasing, with 196,000 procedures performed in the United States in 2015.1 Of those, 24 percent involve anatomic alteration of the native gastrointestinal conduit, such as with RYGB. UGIB occurs with an incidence of 50 to 100 per 100,000 individuals per year in the United States, causing an estimated 20,000 deaths annually.6 Gastric malignancy, while typically a less common cause of UGIB, remains the fifth most common cancer worldwide and second leading cause of cancer death.7,8 With an aging population and increased performance of bariatric surgeries, there is a cumulative increased risk of development of gastric adenocarcinoma in the excluded gastric remnant. What makes this pathology particularly challenging is the relative inaccessibility of this malignancy endoscopically.
Endoscopic access to the excluded stomach after RYGB remains technically challenging, and this impairs diagnosis, management, and surveillance of UGIB in this population. First-line treatment for hemodynamically stable UGIB is resuscitation followed by endoscopy, with surgical intervention reserved for acute hemodynamic instability or UGIB refractory to maximal medical therapy. Although rarely reported in the literature (six reports of gastric malignancy diagnosed in an excluded gastric remnant after gastric bypass),9-14 the prevalence of gastric malignancy in the remnant stomach will undoubtedly increase with an aging population and with continued performance of weight loss surgery with in situ gastric remnants.
Diagnostic and minimally invasive interventional procedures reported in the literature to evaluate gastric remnant pathology include double balloon-assisted enteroscopy or laparoscopic-assisted gastroduodenoscopy with/without endoscopic retrograde cholangiopancreatography (ERCP).2-5,15 These are advanced techniques that require specialized equipment and skilled endoscopists, bariatric surgeons, and advanced laparoscopic surgeons, which may not be easily accessible at all hospitals. Knowledge of the postsurgical gastrointestinal anatomy and the technical skills to navigate this anatomy is imperative for patient safety and successful operative outcomes.
This case highlights the techniques for minimally invasive management of UGIB in patients with in situ gastric remnants and the importance of having a low index of suspicion for malignancy in patients presenting with medically refractory UGIB who have in situ gastric remnants. Difficulty or delay in accessing the gastric remnant may prolong bleeding and time to accurate diagnosis. Preoperative endoscopic or intraoperative laparoscopic-assisted endoscopic localization of the bleeding source is imperative prior to surgical resection and facilitates both minimally invasive management of UGIB and adequate oncologic surgical procedures in the case of a discovered malignancy. What is unknown at present is the oncologic implications of performing an intraoperative gastrotomy to facilitate laparoscopic-assisted endoscopic diagnosis of a gastric cancer. Recent literature evaluating emergency gastric perforations with malignant etiology has disproven the theory of worsened peritoneal dissemination and carcinomatosis with gastric perforation. Recent literature has also reported that the stage of the malignancy is more correlated with survival than with the perforation status.16 The appropriate screening or surveillance of patients at high risk of gastric malignancy, such as those with certain ethnic backgrounds or hereditary syndromes, remains to be determined.8,15
Conclusion
Acute UGIB in a patient with an in situ gastric remnant after bariatric surgery requires preoperative localization of the bleeding source, utilizing endoscopic or laparoscopic-assisted endoscopic techniques to visualize the gastric remnant. A low threshold of suspicion should be maintained for malignancy, especially as the United States population is aging and bariatric surgery is being increasingly performed. If bleeding from the excluded stomach is identified, endoscopic and/or surgical management is reasonable, provided the institution has appropriate resources and technical expertise. The preoperative bariatric surgery evaluation and counseling should include an assessment of risk factors for gastric adenocarcinoma.
Lessons Learned
Evaluation of the gastric remnant in patients with an UGIB and who have undergone RYGB remains a technically challenging but imperative endeavor. Endoscopic or laparoscopic-assisted techniques are available for localizing the bleeding source, and a suspicion of malignancy should always be maintained.
Authors
Molly W. Meyers, MD
Northwestern Memorial Hospital, Department of Surgery, Chicago, IL
Juaquito M. Jorge, MD
Northwestern Memorial Hospital, Department of Surgery, Chicago, IL
Alexander P. Nagle, MD, FACS
Northwestern Memorial Hospital, Department of Surgery, Chicago, IL
Correspondence Author
Dr. Alexander P. Nagle
Department of Surgery
251 E. Huron St., Galter 3-150
Chicago, IL 60611
312-695-8918
Alexander.Nagle@nm.org
Author Affiliations
Northwestern Memorial Hospital, Department of Surgery, Chicago, IL 60611
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011-2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 10, 2018.
- Baron TH, Vickers SM. Surgical gastrostomy placement as access for diagnostic and therapeutic ERCP. Gastrointest Endosc. 1998;48(6):640-641.
- Bertin PM, Singh K, Arregui ME. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography (ERCP) after gastric bypass: case series and a description of technique. Surg Endosc. 2011;25(8):2592-2596.
- Bowman E, Greenberg J, Garren M, et al. Laparoscopic-assisted ERCP and EUS in patients with prior Roux-en-Y gastric bypass surgery: a dual-center case series experience. Surg Endosc. 2016;30(10):4647-4652.
- Gutierrez JM, Lederer H, Krook JC, et al. Surgical gastrostomy for pancreatobiliary and duodenal access following Roux en Y gastric bypass. J Gastrointest Surg. 2009;13(12):2170-2175.
- El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154-1158.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Stomach Cancer. https://seer.cancer.gov/statfacts/html/stomach.html. Accessed April 10, 2018.
- Choi IJ. Endoscopic Gastric Cancer Screening and Surveillance in High-Risk Groups. Clin Endosc. 2014;47(6):497-503.
- Raijman I, Strother SV, Donegan WL. Gastric cancer after gastric bypass for obesity. J Clin Gastroenterol. 1991;13:191-194.
- Lord RV, Edwards PD, Coleman MJ. Gastric cancer in the bypassed segment after operation for morbid obesity. Aust NZ J Surg. 1997;67(8):580-582.
- Khitin L, Roses RE, Birkett DH. Cancer in the gastric remnant after gastric bypass: a case report. Curr Surg. 2003;60(5):521-523.
- Corsini DA, Simoneti CAM, Moreira G, et al. Cancer in the excluded stomach 4 years after gastric bypass. Obes Surg. 2006;16(7):932-934.
- Escalona A, Guzman S, Ibanez L, et al. Gastric cancer after Roux-en-Y gastric bypass. Obes Surg. 2005;15(3):423-427.
- Watkins BJ, Blackmun S, Kuehner ME. Gastric adenocarcinoma after Roux-en-Y gastric bypass: access and evaluation of excluded stomach. Surg Obes Relat Dis. 2007;3(6):644-647.
- Evans JA, Muthusamy VR, Acosta RD, et al. The role of endoscopy in the bariatric surgery patient. Gastrointest Endosc. 2015;81(5):1063-1072.
- Kasakura Y, Ajani JA, Mochizuki F, et al. Outcomes after emergency surgery for gastric perforation or severe bleeding in patients with gastric cancer. J Surg Oncol. 2002;80(4):181-185.