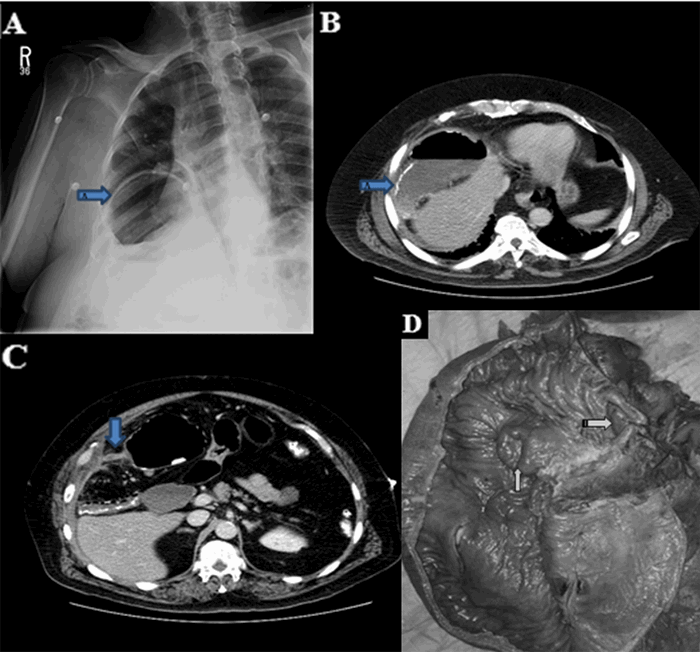
Figure 4. Esophagogastroduodenoscopy displaying stent placement over right-sided esophageal blood clot (arrow).
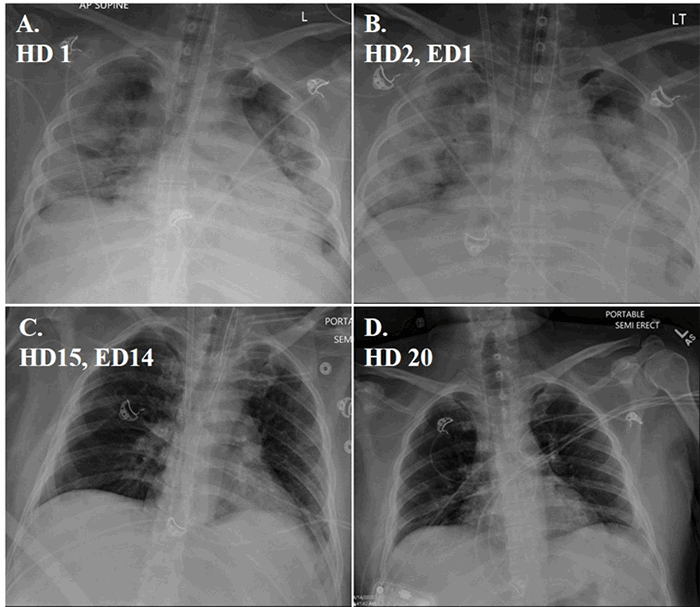
Post-EGD, CT surgery was consulted for operative correction of fistula, but surgery was cancelled after patient suffered an ST-elevation myocardial infarction from air emboli entering the coronary vasculature while awaiting emergent exploration. Despite efforts to limit neurologic damage, the patient's neurologic and hemodynamic status continued to decline, and his family decided to withdraw care.
Discussion
An atrial-esophageal fistula (AEF) is defined as an aberrant anastomosis connecting the atrial cavity to the esophageal lumen. AEF requires an insult, spanning all histologic layers, between the atrial wall and the esophageal wall in order to form. Most common insults include trauma (gunshot wound, blunt penetration), surgical incisions or cautery, and systemic inflammatory disease (such as inflammatory bowel disease). Most patients present three to four weeks after the initial insult with sepsis from esophageal flora entering the blood through the fistula.1 Patients can also present with neurologic deficits from air emboli entering the circulation during peristalsis.1 As in this case, emergent exploration is often delayed in patients presenting with cerebrovascular accident symptoms due to time to image and identify the sources of air emboli. Air emboli can quickly progress to diffuse pneumocephalus requiring urgent endoscopic or surgical intervention to limit neurologic damage. Often, endoscopy will visualize a clot in the esophagus at the fistula site and the presence of old blood in the stomach from blood leaking through the fistula. Esophageal stents can be placed to temporarily occlude the fistula until surgical correction can be performed, but stents should be generally understood as a temporary measure until surgery and not a definitive treatment.2 Furthermore, endoscopy poses a risk of worsening air emboli due to insufflation; however, in order to do so, insufflation pressure must overcome luminal fistula pressures. Therefore, endoscopic treatment of AEF should limit insufflation time and be reserved for skilled endoscopists.3 AEF survival outcomes significantly favor surgery over esophageal stenting, which makes quickly identifying fistulas and resuscitating the patient for surgery of utmost importance.4 Surgical correction typically includes cardiopulmonary bypass with open atrial repair to limit and excise tissue necrosis, followed by esophageal repair or resection for fistula resolution.5,6 Regardless of initial treatment option, early time to diagnosis and treatment improves survival. Without endoscopic or surgical intervention, mortality rates of 83 percent have been reported.1
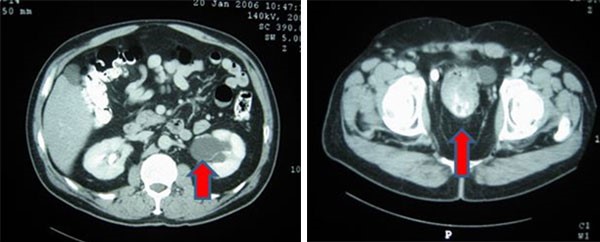
Maze catheter ablation is a minimally-invasive surgical procedure that utilizes an energy source, such as microwaves, radiofrequencies, or cryothermy, to create a superficial scar on the heart to block conduction from pathologic electrical foci. Radiofrequency ablation is often chosen over other energy sources for the correction of atrial fibrillation due to radiofrequency being reported to have a lower rate of atrial fibrillation recurrence.7 Maze procedures for the treatment of atrial fibrillation have reported a small risk of AEF due to the proximity of the esophagus to the atrial cavities. Literature has reported an AEF incidence in maze procedures (depending on the energy source) of 0.03 to one percent. 1,2 Often, a diagnosis will be made with the use of chest CT with and without contrast displaying the presence of a fistula. Less commonly, contrast-enhanced echocardiography can make a diagnosis by visualizing air bubbles prior to saline agitation. This case demonstrates a rare complication of maze radiofrequency catheter ablation and how swiftly a patient can deteriorate from AEF if emergent surgery isn't performed in time. Esophageal stents and surgery can hopefully limit more air emboli from entering circulation, but cannot prevent present emboli from causing catastrophic events like myocardial infarction, as in this patient. The presence of an AEF diagnosis should be treated as an emergency, with a primary focus on quickly resuscitating the patient for surgery.
Conclusion
Atrial-esophageal fistula is a complication of maze radiofrequency catheter ablation that can result in high mortality if not urgently corrected. Patients can present with an array of symptoms (with sepsis being the most common presentation). Emergent surgical intervention is necessary to provide the highest likelihood of survival.
Lessons Learned
Fistulae connecting the gastrointestinal tract to the bloodstream have high percentages of morbidity and mortality. There is limited time for survival in AEF patients presenting with signs of sepsis or stroke, whose workup often inadvertently delay time-to-surgery. Resuscitating AEF patients for surgical intervention is of utmost importance to improve survival outcomes. CT surgery should be immediately involved with patient care at time of AEF identification.
Authors
Eric M. Montminy, MD
Tulane University School of Medicine, Department of Internal Medicine, New Orleans LA
Vanessa Mendez, MD
Tulane University Medical Center, Department of Gastroenterology, New Orleans, LA
Christopher G. Ducoin, MD, FACS
Tulane University Medical Center, Department of Surgery, New Orleans, LA
Correspondence
Dr. Eric Montminy
Internal Medicine Resident
Tulane University Medical School of Medicine
1430 Tulane Avenue, SL-50
New Orleans, LA 70112
Telephone: 1-630-306-0555
Email: emontmin@tulane.edu
Fax: 1-504-988-3971
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
Rohit Maini, MD; and Amy Scheller, MD; both contributed to obtaining imaging.
Meeting Presentation
Case was presented at the World Congress of Gastroenterology/American College of Gastroenterology 2017 National Conference, Orlando, FL October 2017
References
- Chavez P, Messerli F, Casso Dominguez A, et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systemic review of case reports. Open Heart. 2015;2(1):e000257
- Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247-55.
- Nair K, Danon A, Valaparambil A, et al. Atrioesophageal fistula: a review. J Atr Fib. 2015;8(3):1331. doi:10.4022/jafib.1331
- Singh SM, d'Avila A et al. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm. 2013;10(11):1591-97.
- Hartman A, Glassman L, Katz S, Chinitz L, Ross W. Surgical repair of a left atrial-esophageal fistula after radiofrequency catheter ablation for atrial fibrillation. Ann Thorac Surg. 2012;94(4):e91-3. doi:10.1016/j.athoracsur.2012.04.052.
- Chavez P, Messerli F, Dominguez A et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: a systemic review of case reports. Open Heart 2015;2:e000257. doi:10.1136/openhrt-2015-000257
- Phan K, Xie A, Kumar N et al. Comparing energy sources for surgical ablation of atrial fibrillation: a Bayesian network meta-analysis of randomized, controlled trials. Eur J Cardothorac Surg. 2015;48:201-11.