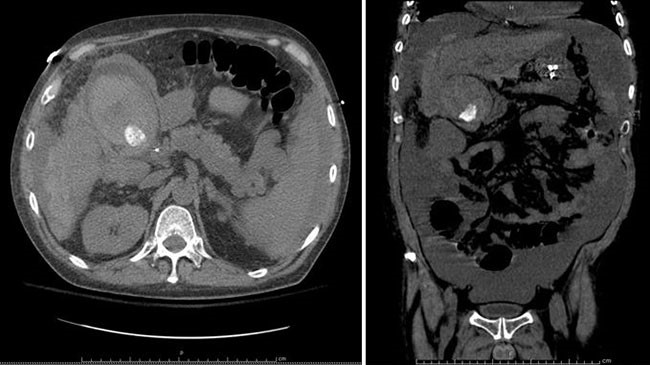
Figure 2. Coronal image of worsening diverticulitis involving the descending and sigmoid colon.
No drainable fluid collections were noted. There was also new thickening of the distal transverse colon, cecum, and ascending colon, interpreted by radiology as superimposed colitis versus new diverticulitis. These new findings prompted C. difficile stool studies to be obtained. Infectious disease (ID) consultation was obtained and recommended IV antibiotic coverage changed to ampicillin/sulbactam for better entercoccal coverage. Over the next few days, the patient improved clinically, her WBC counts trended down (13.3 k/uL), and repeat CT abdomen and pelvis on hospital day five demonstrated overall no change, with diffuse wall thickening of ascending, transverse, descending, and sigmoid colon. Per ID recommendations, antibiotics were adjusted to oral amoxicillin/clavulanate.
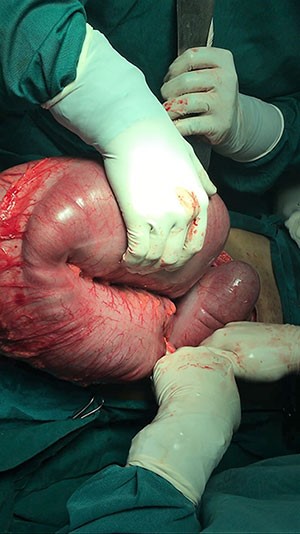
Subsequently, the patient began having diarrhea, febrile episodes, and C. difficile PCR stool studies returned positive. The patient was started on oral metronidazole, but continued her febrile episodes and rising WBCs (33.6 k/uL). Oral vancomycin was added to the patients' antibiotic regimen. The patient was transferred to the intensive care unit (ICU) for close monitoring. The patient continued to develop fulminant C. difficile infection (CDI) colitis as she remained febrile, with elevated white cell count (37.6 k/uL), and lactate (8.7 mmol/L). The decision was made to take the patient to the operating room emergently. The patient underwent a diagnostic laparoscopy that was converted to exploratory laparotomy, lysis of adhesions, and diverting loop ileostomy with intraoperative colonic lavage with high-volume polyethylene glycol. Intraoperatively, the entire colon was inspected without evidence of ischemia or necrosis. A total of 8 liters of polyethylene glycol 3350/balanced electrolyte solution was instilled intraoperatively via the efferent ileum through a 24 French Malecot catheter. A rectal tube placed preoperatively was inspected during the installation; it was noted that the brown formed stool became clear. The patient return to the ICU intubated in critical condition. Postoperatively, antegrade instillation of vancomycin 500 mg in 500 mL via the ileostomy every six hours for a total of ten days was performed. ID recommended continued IV metronidazole in conjunction with the vancomycin flushes. Fidaxomicin was added to the vancomycin lavages. On postoperative day two, gastroenterology performed a fecal transplantation from a standard donor acquired from an outside institution using five vials each containing 60 mL through the patient's efferent loop of the ileostomy via the Malecot catheter. The patient subsequently developed acute respiratory distress syndrome needing high oxygenation requirements. The patient eventually underwent low tidal volume protocol with prone positioning. Vancomycin lavages were completed, and the patient clinically improved. The patient underwent tracheostomy with weaning and discontinuation of mechanical ventilation. The remaining hospital course was uneventful, and the patient was discharged to a skilled nursing facility for rehabilitation on hospital day 30. The patient returned for reversal of her diverting loop ileostomy, which was preformed seven months later.
Discussion
In the past decade, CDI has become one of the major causes of nosocomial diarrhea, and this condition displays a full spectrum of symptoms that range from asymptomatic to fulminant colitis with systemic toxicity and death.2-5 There are several risk factors for the development of CDI, including advanced age, immunodeficiency, health care employment, recent hospitalization, recent gastrointestinal surgery, and, in particular, antimicrobial exposure.3,4,7 In 1978, antibiotic use was established as the strongest risk factor, with up to 98 percent of patients having had at least one dose of antibiotics in the preceding two weeks.1,3,4
CDI was previously considered a hospital-acquired infection; however, recent data suggest that community-acquired infections now account for up to 41 percent of cases.1-3 There continues to be a considerable increase in the incidence and severity of CDI colitis. United States national database reporting demonstrated a 2 to 2.5-fold increase from the 1990s to the early 2000s.2,4,5 Oral antibiotic therapy is successful in treating a majority CDI, but 3 to 10 percent of CDI cases will progress to severe, complicated, or fulminant systemic toxicity.5,7,8
Most medical guidelines recommend surgical intervention to treat complicated and fulminant CDI.8 Clinical indications such as worsening abdominal exams, peritonitis, and systemic shock indicate surgical intervention.5,7,8 When nonoperative management has failed in critically ill patients, total abdominal colectomy with end ileostomy has been advocated as the operation of choice.5,8-10 The benefits of this approach include minimizing the duration of surgery in critically ill patients, better handling of the enlarged and edematous colon, and decreasing the chance of abdominal compartment syndrome, which can be precipitated by laparoscopic insufflation.9 Decreased survival has been reported in patients treated with segmental colectomy likely attributed to CDI affecting the entire colon.10 However, the associated mortality of total abdominal colectomy remains high, with only marginal improved survival compared to nonoperative management.2,5,7-9,11 The mortality for total abdominal colectomy for CDI ranges from 35 to 80 percent.2,5,11-14 Subtotal and total colectomy additionally has significant morbidity for survivors, including permanent ileostomy.2,5,15
As CDI incidence continues to increase, the surgical treatment for moderate to severe CDI remains debated.2,15-18 Given the pathophysiology of C. difficile's endotoxin-mediated mucosal toxicity and inflammation, the emergence of the diverting loop ileostomy with intraoperative colonic lavage of high-volume polyethylene glycol, followed by postoperative antegrade instillation of vancomycin flushes via the ileostomy, proposes a less morbid approach to treating this disease.8,15 This technique, described in a case-control series by Neal et al from the University of Pittsburgh, is promising, but limited by the small cohort, retrospective comparison, and lack of randomization.9,15 However, this minimally invasive technique is a less morbid procedure with a long-term benefit of colonic preservation, representing a future treatment option early in the disease course.9,14
Recently, fecal microbiota transplantation (FMT) has been described as a novel, highly effective intervention in patients with recurrent or refractory C. difficile infection.19-22 FMT has been found to treat refractory CDI with an 81 percent resolution rate.18 The literature is scarce in regards to treatment of fulminant CDI with fecal transplantation.
Our patient was treated with diverting loop ileostomy with intraoperative colonic lavage of high-volume polyethylene glycol, followed by postoperative vancomycin flushes via the ileostomy, and in addition to postoperative FMT.
Conclusion
We report a single case of sigmoid diverticulitis with superimposed development of CDI colitis, and progression to fulminant CDI, requiring emergent surgical intervention. CT demonstrating diffuse persistent pancolitis, increasing WBCs, and positive C. difficile stool toxin, was initially treated conservatively. Nonoperative treatment was initiated with oral metronidazole and escalated to oral vancomycin without clinical improvement. Traditional surgical interventions result in total abdominal colectomy with end ileostomy, which has been found to have mortality and marginal improved survival. Recent clinical trials validated the development of the diverting loop ileostomy and intraoperative colonic lavage with high-volume polyethylene glycol followed by postoperative antegrade instillation of vancomycin flushes via the ileostomy to preserve the colon. In this case, a diverting loop ileostomy with colonic lavage was complimented with postoperative fecal transplantation via the diverting loop ileostomy. With the increasing incidence of C. difficile colitis and newly developing hypervirulent strains, it is imperative to continue developing newer protocols to treat evolving bacterial infections.
Lessons Learned
C. difficile infection is a serious and increasingly problematic health care issue. When surgical intervention is required, perhaps less morbid colon preserving techniques should be performed with a multimodal approach, including postoperative fecal transplantation.
Authors
Robert T. Nevitt III, MD
Mercy Catholic Medical Center, Department of Surgery, Darby, PA
John B. Fobia, MD, FACS
Mercy Catholic Medical Center, Department of Surgery, Darby, PA
Correspondence
Dr. Robert T. Nevitt III
Mercy Catholic Medical Center
Department of Surgery
1500 Lansdowne Ave, MS82
Darby, PA 19023
(610) 237-4960
nevitt.robert@gmail.com
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Bartlett J, Moon N, Chang T, et al. Role of Clostridium difficile in antibiotic associated pseudomembranous colitis. Gastroenterology 1978;75:778-782.
- Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann
Surg. 2002;235(3):363-372. - Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-16.
- Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269(1):71-5.
- Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: Patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-9.
- Berman L, Carling T, Fitzgerald T. Defining Surgical Therapy for Pseudomembranous Colitis with Toxic Megacolon. J Clin Gastroenterol. 2008;42(5):476-480.
- Longo WE, Mazuski JE, Virgo KS, et al. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47(10):1620-6.
- Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for health-care epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
- Kaiser, AM, Hogen R, Bordeianou L, Alavi K, Wise PE, Sudan R. Clostridium Difficile Infection from a Surgical Perspective. J Gastrointest Surg. 2015;19:1363-1377.
- Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Am J Gastroenterol. 2013;108:478-498.
- Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196(3):384-8.
- Dudukgian H, Sie E, Gonzalez-Ruiz C, et al. C. difficile colitis—predictors of fatal outcome. J Gastrointest Surg. 2010;14(2):315-322.
- Byrn JC, Maun DC, Gingold DS, et al. Predictors of mortality after colectomy
for fulminant Clostridium difficile colitis. Arch Surg. 2008143(2):150-4. - Synnott K, Mealy K, Merry C, et al. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85(2):229-231.
- Neal, MD, Alverdy, JC, Hall, DE, Simmons, RL, Zuckerbraun,BS. Diverting Loop Ileostomy and Colonic Lavage: An Alternative to Total Abdominal Colectomy for the Treatment of Severe, Complicated Clostridium difficile Associated Disease. Ann Surg. 2011;254(3):423-9.
- Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079-1084.
- Eggertson L. C. difficile strain 20 times more virulent. CMAJ. 2005;172(10):1279.
- Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-9.
- Nood EV, Vrieze A, Nieuwdorp M, et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med. 2013;368(5).
- Johannes A, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: Case series involving 18 patients treated with donor stool administered via a Nasogastric tube. Clin Infect Dis. 2003;36:580-5
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994-1002.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142(3): 490-6.