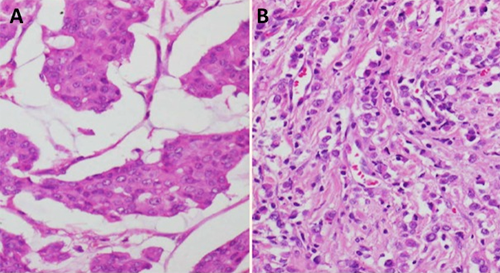
Figure 2. Histopathology slides. A: Ductal carcinoma breast , left breast. B: Lobular carcinoma breast, right accessory breast
Discussion
Accessory breasts occur in 0.22 to 6 percent of the general population and can occur anywhere along the milk line.1 These are most commonly seen in the axilla, which accounts for 60 to 70 percent of ectopic breast tissues.4,5 While breast cancer is the most common malignancy in women, those arising in accessory breast constitute only 0.3 to 0.6 percent of total breast cancers.3
Common tumours presenting in the axillary accessory breast include benign lesions like fibroadenomas, or malignancies like primary mammary carcinoma, lymphoma, or skin appendageal tumour.6,7 These usually present with lump or skin changes. In a review of ectopic breast cancers done by Yanagi et al, skin changes in the form of discoloration, depression, and ulceration were seen in 49 percent of patients.8
Most of the women with axillary accessory breast are symptomatic at presentation, as these are often missed on screening mammography due to their peculiar location. Oblique and exaggerated cranio-caudal view can help in the visualization of axillary breast tissue.9 Ultrasonography and MRI are helpful in radiological characterization of the lesion and may help in excluding other common lesions. These are usually seen as hypoechoic lesions on USG, interspersed within echogenic normal breast parenchyma in axillary location, and discontinuous with normal breast tissue. Imaging of the breast with mammogram or MRI should also be done to identify any primary breast cancer.
Diagnosis of this tumour type is often delayed because they rank low in clinical suspicion and are often confused with an axillary lymphadenopathy or skin appendigeal tumour. Definitive diagnosis depends on histopathology of the lesion demonstrating malignant ductal cells surrounded by normal breast parenchyma with absence of lymphoid tissue. Immunohistochemistry can further confirm their mammary origin. Mammary glandular tissue interspersed amongst adnexal gland suggests an accessory axillary breast tissue rather than normal breast. The most common histological type is infiltrating ductal carcinoma.10 In a review by Marshall et al, invasive ductal carcinoma was seen in almost 80 percent cases, while medullary and lobular carcinoma comprised the remainder.5
Due to the rare occurrence of such cancers, no definitive diagnostic, staging, or treatment protocols exist for management of tumours arising from accessory breast. There has been a shift from radical mastectomy to wide local excision in accessory breast cancer management. Cogsweel & Czerny studied the autopsy results of patients who died of metastasis from accessory breast tissue and found that even in the presence of metastasis, ipsilateral breast did not show presence of invasive breast cancer, making wide local excision a feasible option in these cases.11 Subsequently various articles have suggested that the surgical procedure of choice for ectopic breast carcinoma should be a wide resection of the tumour with surrounding normal tissue, including the skin and regional lymph nodes.12 Zhang et al stated in their paper that "when the accessory breast tissue is closely connected to normal breast tissue, the indications for surgery should be the same as those for a tumour anatomically situated in the breast." Likewise, there are also no standard protocols for axillary management in these cases. While routine axillary lymph nodal management was previously followed in view of the understanding that axillary lymph nodes are more often involved, SLNB is now being increasingly adopted. In the review by Zang et al, it was seen that lymph node positivity was similar between accessory and usual breast cancers when compared stage to stage.8 Although the indications for adjuvant chemotherapy and hormonal therapy remains the same, the need for irradiating the normal ipsilateral breast after wide local excision remains controversial.
The prognosis of such tumours is difficult to characterize due to limited data on such cases and absence of recommended TNM staging. While some authors consider similar prognosis and prognostic characteristics as with mammary carcinoma, others believe that they have a worse prognosis due to close proximity and increased axillary lymph nodal involvement. Further data is needed to ascertain the prognostic variables for this entity.13
Our case is unusual for more than one reason: it represents bilateral disease, with right-sided disease arising within an axillary accessory breast, and left-sided disease arising in the normal pectoral breast tissue. Each of these had differing histologies. Both the tumours were amenable for WLE and SLNB as per standard protocols for breast cancer management.
Conclusion
Cancer arising in accessory breast tissue is rare, and a high clinical suspicion is required for a timely diagnosis. Wide local excision with sentinel lymph node biopsy is feasible in appropriately selected cases.
Lessons Learned
Cancer arising in accessory breast tissue is extremely rare, and a high clinical suspicion is required for a timely diagnosis. Histologically, diagnosis is made by having focus of malignant cells with interspersed breast epithelia in the absence of lymphoid tissue. Evaluation of bilateral breast in every case of breast carcinoma remains of prime importance to rule out a second primary tumour or a tumour in the opposite breast. Wide local excision with SLNB is feasible in appropriately selected cases.
Authors
Suhani, MBBS, MS, DNB, FACS
Assistant Professor, Department of Surgical Disciplines, All India Institute of Medical Sciences, New Delhi
Vuthaluru Seenu, MBBS, MS, FACS
Professor and surgical unit head, Department of Surgical Disciplines, All India Institute of Medical Sciences, New Delhi
Rajinder Parshad, MBBS, MS, DNB
Professor and surgical unit head, Department of Surgical Disciplines, All India Institute of Medical Sciences, New Delhi
Smriti Hari MBBS, MD
Professor, Department of Radio diagnosis, All India Institute of Medical Sciences, New Delhi
Sandeep Mathur, MBBS, MD
Professor, Department of Pathology, All India Institute of Medical Sciences, New Delhi
Haresh KP, MBBS, MD
Associate Professor, Department of radiation oncology, BRAIRCH, All India Institute of Medical Sciences, New Delhi
Correspondence
Dr. V. Seenu
Room number 5026, Teaching Block
Department of Surgical Disciplines, AIIMS, New Delhi.
Email: varnaseenu@hotmail.com
Phone: 91- 9910834455
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Scanlan KA, Propeck PA. Accessory breast tissue in an unusual location. AJR Am J Roentgenol. 1996;166:339-340. doi: 10.2214/ajr.166.2.8553942
- Devine C, Courtney CA, Deb R, Agrawal A. Invasive lobular carcinoma arising in accessory breast tissue. World J Surg Oncol. 2013;11:47. doi: 10.1186/1477-7819-11-47
- Yamamura J, Masuda N, Kodama Y, Yasojima H, Mizutani M, Kuriyama K, Sekimoto M. Male breast cancer originating in an accessory mammary gland in the axilla: A case report. Case Rep Med. 2012:286210
- Evans, D. M. and D. P. Guyton . Carcinoma of the axillary breast. J Surg Oncol. 1995;59:190-195.
- Marshall MB1, Moynihan JJ, Frost A, Evans SR. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994 Oct;3(5):295-304.
- Oshida K, Miyauchi M, Yamamoto N, Takeuchi T, Suzuki M, Nagashima T, et al. Phyllodes tumour arising in ectopic breast tissue of the axilla. Breast Cancer 2003;10:82-4.
- Amsler E, Sigal-Zafrani B, Marinho E, Aractingi S. Ectopic breast cancer of the axilla. Ann Dermatol Venerol. 2002;129:1389-91.
- Zhang S, Yu Y-H, QU W, Zhang Y, LI J. Diagnosis and treatment of accessory breast cancer in 11 patients. Oncology Letters. 2015;10(3):1783-1788. doi:10.3892/ol.2015.3388.
- Nihon-Yanagi Y, Ueda T, Kameda N et al: A case of ectopic breast cancer with a literature review. Surg Oncol, 2011;20: 35-42.
- Madej B, Balak B, Winkler I, Burdan F. Cancer of the accessory breast - a case report.Adv Med Sci. 2009;54:308-310. doi: 10.2478/v10039-009-0031-6
- Cogswell H, Czerny EW. Carcinoma of aberrant breast of the axilla. Am Surg. 1961;27:388-390.
- Livesey JR, Price BA. Metastatic accessory breast carcinoma in a thoracic subcutaneous nodule. J R Soc Med. 1990;83:799-800.
- Ahmed M, Aurangzeb, Pervez A, et al: Primary carcinoma of ectopic breast tissue in axilla. J Coll Physicians Surg Pak. 2012;22:726-727.