A Minimally Invasive Approach to Persistent Gastric Staple-Line Failure
April 24, 2020
Abstract
Background
A female patient presented fourteen days after undergoing laparoscopic sleeve gastrectomy for weight loss with gastric staple-line failure.
Summary
A 37-year old morbidly obese female presented postoperative day 14 after an uneventful laparoscopic sleeve gastrectomy. On CT imaging, she was found to have gastric staple-line failure near the gastroesophageal junction with a contained leak. The patient was placed on bowel rest, antibiotics, and parenteral nutrition without symptom improvement. The patient developed intraabdominal sepsis and enlarging fluid collection on imaging. Repeat upper gastrointestinal (UGI) contrast study demonstrated continued leak at the gastric staple line. The patient underwent laparoscopic-assisted percutaneous endoscopic gastrostomy (PEG) tube placement through the perforation in order to promote maturation of fistula tract and enteric drainage. The surgical drain was left for control of leak and management of intraabdominal sepsis followed by laparoscopic jejunostomy tube placement for distal enteral feeding. The PEG tube was ultimately removed after assuring closure of leak on UGI following eight weeks of adequate enteral nutrition.
Conclusion
Gastric staple-line failure can be managed in many ways. Persistent failure, despite bowel rest, often results in invasive surgical repair and drainage, with the potential for significant associated morbidity and mortality. This case presentation highlights a method of successful minimally invasive control of this difficult problem.
Key Words
Gastric staple-line failure, gastric staple-line leak
Case Description
Laparoscopic sleeve gastrectomy has become a feasible and favorable option in the surgical management of morbid obesity.1 Unfortunately, gastric staple-line failure with intraabdominal contamination remains a difficult to manage complication with associated morbidity and even mortality.2 Preferred methods of management continue to evolve as these complications are more widely discussed in the surgical literature, often based on patient presentation and severity of illness related to intra-abdominal sepsis. Here we report a minimally invasive approach to persistent gastric staple-line failure after a failed trial of conservative management.
The patient, a 37-year-old morbidly obese female with a history of diabetes mellitus, hyperlipidemia, hypertension, and gastroesophageal reflux disease, presented after a reported uneventful laparoscopic sleeve gastrectomy fourteen days previously. She reported persistent nausea, vomiting, and slowly progressive abdominal pain since the time of her operation, prompting evaluation. Initial evaluation was carried out with contrasted UGI study that demonstrated a small leak at the proximal aspect of her gastric staple-line. Conservative management was initiated with bowel rest and parenteral nutrition.
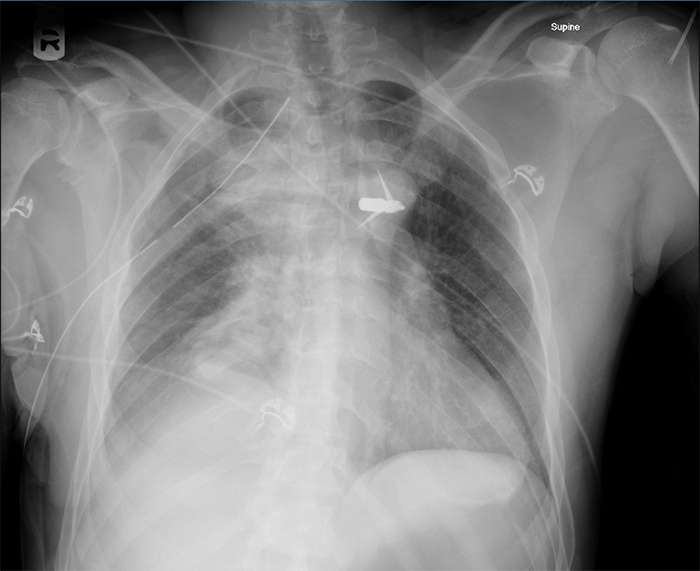
After three weeks of conservative management, the patient returned with persistent symptoms of nausea, vomiting, abdominal pain, and fever. CT imaging demonstrated evidence of continued proximal staple-line leak with extraluminal air (Figure 1).
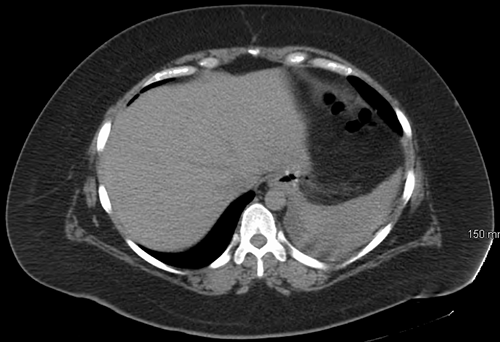

Figure 1. Proximal staple-line leak with extraluminal air
The patient was admitted for a period of observed oral restriction due to concern for lack of adherence to bowel rest. UGI was then performed to assess evidence of continued leak, confirming persistence of previously diagnosed area of staple-line failure (Figure 2).

Figure 2. Persistent gastric staple-line leak at gastroesophageal junction
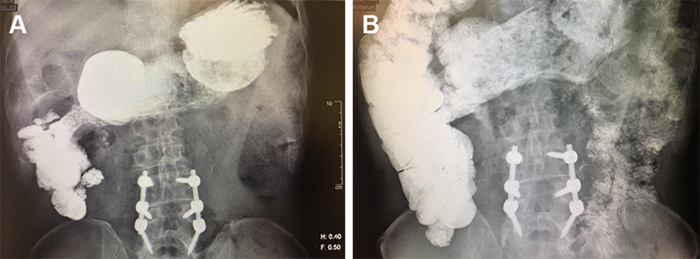
Based on persistence of leak, intraabdominal abscess formation, and the lack of enteral feeding access, a plan was made for operative intervention. The patient was taken to the operating room for laparoscopic assisted PEG tube placement after endoscopic and laparoscopic confirmation of the suspected location of staple-line failure. This area was traversed with a 20Fr externally removable PEG tube, leaving 20 cm of redundant tubing without tension to allow the leak area to become a mature fistula tract. Laparoscopic jejunostomy tube placement was performed for reliable distal feeding access, and a surgical drain was left along the staple line in the area of abscess formation (Figure 3).
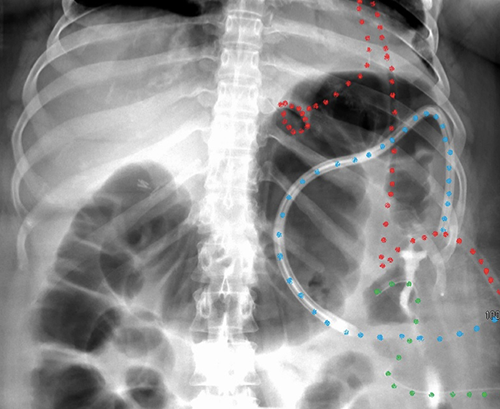
Figure 3. Postoperative imaging with color-coded tubes (red = PEG, blue = drain, green = J tube)
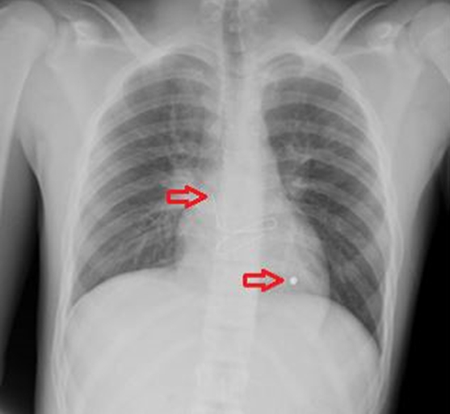
The surgical drain was removed after output decreased to a negligible amount. Surveillance CT scan imaging was performed at return clinic visit to discuss PEG tube removal (Figure 4).
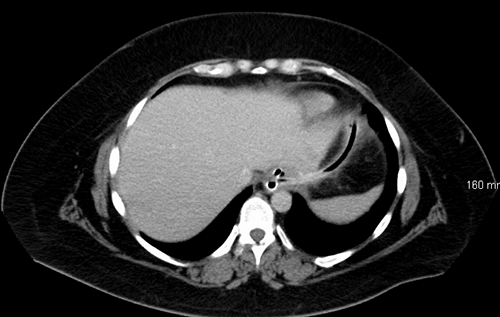
Figure 4 PEG tube in fistula tract prior to removal
EGD was then performed following improvement in the patient's nutritional status to facilitate PEG tube removal, followed by UGI study to evaluate the fistula tract (Figure 5).
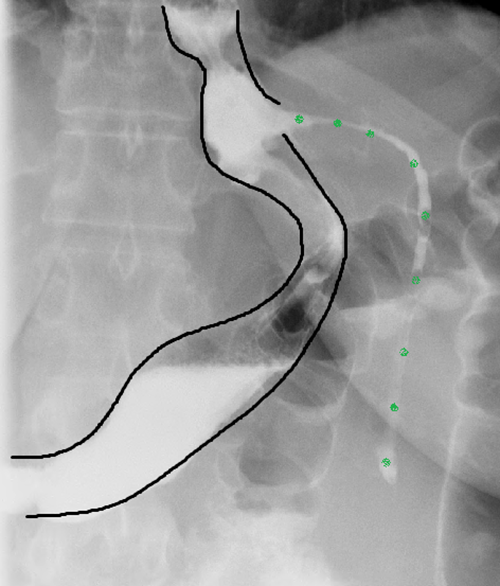
Figure 5. Mature fistula tract after tube removal (green = fistula tract)
The patient was allowed to increase oral intake in addition to jejunal feeding. She returned to the clinic one month later, where her fistula tract was noted to be completely healed. Her jejunostomy tube was removed, and she returned to her previously planned postgastrectomy diet. At two-year phone follow-up, the patient denied any fistula recurrence.
Discussion
Complications related to laparoscopic sleeve gastrectomy continue to be reported in the literature. Since its first description in the 1990s as a staged portion of the duodenal switch operation, laparoscopic sleeve gastrectomy has since become a widely accepted alternative to more traditional weight-loss operations.1,3 As this operation continues to gain popularity, the number of patients who suffer procedure-related complications will only increase in number. Management of the acute and potentially life-threatening complications of this operation should be within the realm of the general surgeon; including hemorrhage, staple-line leak, and abscess formation.4 Gastric staple-line leak, specifically, occurs in as many as five percent of patients following sleeve operations and is a potentially devastating complication clinically and logistically.5
Many techniques have been described for the management of gastric staple-line leak after laparoscopic sleeve gastrectomy, each of which focuses on three essential tenets: leak control, drainage of intraabdominal contamination, and maintenance of adequate nutrition. This is often performed in a stepwise fashion dependent on the specific clinical presentation of each individual, ranging from expectant management to repeat operations that may expose the patient to additional morbidity and mortality.
In the early postoperative setting, repeat laparoscopic or open operations for surgical fistula closure, omental patching, or staple-line revision are possible.6 Fortunately or unfortunately, not all leaks will be substantial enough to manifest clinically in a time frame that allows return to the operating room. For this reason, and to attempt to prevent the risk of reoperation, less invasive options have been described, including stenting procedures, with variable results.7 Endoscopic stent placement excludes the fistula in order to allow appropriate healing. Unfortunately, gastric staple-line leak often occurs just beyond the gastroesophageal junction. Stenting at this location may be unsuccessful due to inadequate approximation of the deployed stent and surrounding gastric mucosa. Additionally, stent migration beyond the gastroesophageal junction may occur, resulting in inadequate fistula exclusion. Endoclips may achieve the same goal, but only in the case of small mucosal defects or microperforations.8 In reality, the majority of leaks are not amenable to this intervention, as the surrounding tissues are quite friable, preventing adequate fistula closure.
In the setting of complete staple-line disruption or chronic, refractory fistula, more invasive interventions, are often required. These include Roux-en-Y gastric bypass proximal to leak for diversion, complete excision of the involved viscous via proximal gastrectomy with Roux-en-Y esophagojejunostomy, or gastro-jejunostomy formation with Roux-limb anastomosis directly over the defect, each with favorable results.9-11 Certainly, classic T-tube fistula management may be performed in a minimally invasive fashion, utilizing standard T-tubes or other similar devices. With the addition of an omental patch, this technique has also been reported as successful.12
Perhaps most simply, a trial of nil per os with complete bowel rest may be considered in order to allow time for leak closure; however, one must be careful if this algorithm is chosen, as patient nutrition is critical in promoting healing. A complete listing of the discussed treatment modalities can be found in Table 1.
| Modalities for Treatment of Gastric Staple-line Failure |
| Primary surgical repair:
Fistula exclusion:
Fistula control:
Surgical revision:
Conservative management:
|
Table 1. Listed modalities for treatment of gastric staple-line failure.
Regardless of the chosen management of the leak itself, control of intraabdominal contamination must be assured. Although percutaneous drainage of leaks has been described, these procedures are often inadequate and may not allow isolation or complete control of continued contamination.12 This is often the reason repeat operation is chosen, and is certainly the recommended method of treatment for patients who present in shock secondary to intraabdominal sepsis. In rare cases, conservative management with antibiotics may be attempted as long as closure of leak has been documented with formal contrast evaluation to rule out ongoing drainage.
Perhaps most importantly, the majority of these conservative techniques do not address the need for ongoing nutrition in the face of inadequate healing of staple-line leak. Generally, distal enteral feeding access is felt to be the safest, most reliable method of enteral feeding in the face of ongoing leak. Whether this is obtained with nasoenteral access or surgical feeding, tube placement is often surgeon- and center-dependent. If resources allow for safe nasojejunal tube placement, the patient may be spared an additional operation. Unfortunately, these tubes are fraught with potential problems, including displacement, clogging, patient discomfort, and cosmetic complaints. For this reason, we often recommend surgical jejunostomy placement in the setting of prolonged time to fistula healing. Although this is a more reliable method of maintaining nutrition, it is not without similar complications and requires a return to the operating room.
It is our opinion that the benefit of combined laparoscopic and endoscopic evaluation, as described in this case, allows for appropriate identification of ongoing contamination and location of leak. Beyond that, abscess evacuation and drain placement can be performed concomitantly as well as fistula control and acquisition of feeding access, either endoscopically or surgically. Although this is an isolated report, it allows for the discussion of each of the key tenets of gastric staple-line failure management.
Conclusion
Gastric staple-line failure can be managed in many ways. Persistent failure, despite bowel rest, often results in invasive surgical repair and drainage, with the potential for significant associated morbidity and mortality. This case presentation highlights a method of successful minimally invasive control of this difficult problem.
Lessons Learned
This case highlights the essential tenets of the minimally invasive management of a difficult problem—gastric staple-line leak. Minimally invasive approaches have now been proven successful, and these modalities should be within the armamentarium of all surgeons who care for postoperative laparoscopic sleeve gastrectomy patients.
Authors
Zachary Warriner, MD
Division of Trauma and Surgical Critical Care, LAC+USC Medical Center, University of Southern California, Los Angeles, CA
Levi Procter, MD, FACS
Division of Acute Care Surgical Services, Virginia Commonwealth University, Richmond, VA
Correspondence
Dr. Levi Procter
Assistant Professor of Surgery
Division of Acute Care Surgical Services
Virginia Commonwealth University
1200 East Broad Street
West Hospital, 15th Floor, North Wing
Richmond, VA 23298
levi.procter@vcuhealth.org
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Gumbs AA, Gagner M, Dakin D, et al. Sleeve gastrectomy for morbid obesity. Obes Surg 2007;17:962-969.
- Benedix F, Poranzke O, Adolf D, et al. Staple-line leak after primary sleeve gastrectomy—risk factors and mid-term results: Do patients still benefit from the weight loss procedure? Obes Surg 2017 Jul;27(7):1780-1788.
- Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 2000;10:514-23.
- Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon's guide. Can J Surg 2013;56:347.
- Daskalakis M, Berdan Y, Theodoridou S, Weigand G, Weiner RA. Impact of surgeon experience and buttress material on postoperative complications after laparoscopic sleeve gastrectomy. Surg Endosc 2011; 25:88-97.
- Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, Gutierrez L. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19:1672-1677.
- Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935-939.
- Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC) J Gastrointest Surg. 2013;17:1058-1065.
- Sasson M, Ahmad H, Dip F, Menzo EL, Szomstein S, Rosenthal R. Comparison between major and minor surgical procedures for the treatment of chronic staple-line disruption after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016 Jun;12(5):969-975.
- Vilallonga R, Himpens J, van de Vrande S. Laparoscopic Roux limb placement for the management of chronic proximal fistulas after sleeve gastrectomy: technical aspects. Surg Endosc. 2015 Feb;29(2):414-416.
- Saglam K, Aktas A, Gundogan E, et al. Management of acute sleeve gastrectomy leaks by conversion to Roux-en-Y gastric bypass: a small case series. Obes Surg. 2017 Nov;27(11):3061-3063.
- Court I, Wilson A, Benotti P, Szomstein S, Rosenthal RJ. T-tube gastrostomy as a novel approach for distal staple-line disruption after sleeve gastrectomy for morbid obesity: case report and review of the literature. Obes Surg. 2010 Apr;20(4):519-522.
- Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, et al. Nonsurgical treatment of staple-line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821-826.