Figure 2. Histological and immunohistochemistry findings of the case. A: Haematoxylin and Eosin section (x 100) of colonic biopsies showing infiltrating neoplastic cells (arrow) and signet ring cells (arrow head). B: Duodenal biopsy specimen showing similar looking neoplastic cells (H & E x 400). C: Breast biopsy specimen showing Indian file pattern suggestive of invasive lobular carcinoma (H & E x 400). D: Immunohistochemistry study showing positive uptake for CK-7 E: GCDFP (IHC X 100).
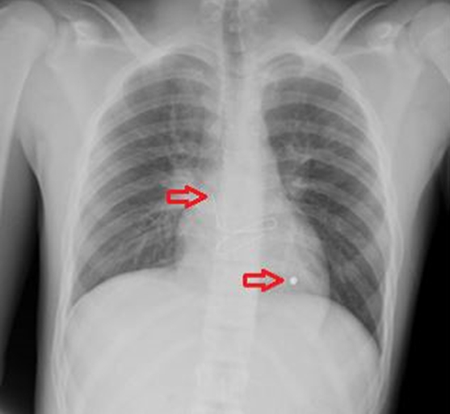
In view of the comorbid illnesses, and the presence of chronic liver disease, the patient was placed on hormonal therapy with tamoxifen.
Discussion
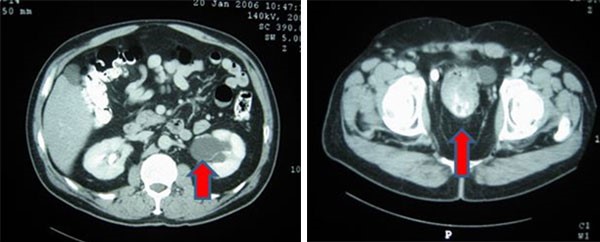
Isolated luminal gastrointestinal metastases from breast carcinoma are rare. Gastrointestinal metastases from breast cancer range from 8 to 35 percent in an autopsy survey. The most common site of gastrointestinal metastases is the stomach (60 percent), followed by the esophagus (12 percent), and colon (11 percent).3,6 Small bowel metastases are still rarer. Multiple-site gastrointestinal metastases, as in our case, have also been reported, but are extremely rare.7
Invasive lobular carcinoma of breast is the most common histological type of breast cancer causing gastrointestinal metastases.8 In one series, 1 in 20 patients with invasive lobular carcinoma were found to have gastrointestinal metastases.7 Gastrointestinal metastases usually occur late following the treatment of primary breast cancer. The median interval from diagnosis of primary tumour to gastrointestinal metastases is about six years (range 0 to 22 years) in literature.3,9 Synchronous metastases as in our case are still rarer.
Primary signet-ring cell carcinoma (SRCC) of the breast is a rare disease, constituting 2 to 4.5 percent of all breast cancers.4 Primary SRCC of the breast and signet-ring cell variant of lobular carcinoma of the breast can be differentiated from the metastatic SRCC by immunohistochemical markers. IHC uses monoclonal as well as polyclonal antibodies to determine tissue distribution of specific antigens of interest. IHC revolutionized the approach to diagnosing tumors of uncertain origin by a using a panel of antibodies based on clinical history, morphological findings, and other investigations.10 IHC markers, importantly GCDFP-15, are positive in primary lesions of breast and negative in gastrointestinal SRCC. CK 7 and ER are positive in primary carcinoma from breast, while CK 20 and CDX2 are usually negative.4,9,11
Treatment of gastrointestinal metastases from breast cancer is usually endocrine therapy and chemotherapy, depending upon the hormonal receptor status of the metastases. Surgical management of the metastatic lesion is usually reserved for complications like obstruction, bleeding, and perforation. Survival of these patients with gastrointestinal metastases from the available data is similar to metastatic breast cancer to other sites; five-year survival is around 29 percent.
Conclusion
Synchronous, multiple-site luminal gastrointestinal metastases from primary breast cancer do occur. Biopsy from the luminal gastrointestinal metastases is likely to show features of signet ring cell carcinoma. Given the high prevalence of breast cancer, it should be considered in any female presenting with new gastrointestinal symptoms and signet-ring cell histological pattern. Immunohistochemistry is the key to solving this diagnostic puzzle and may prevent an unnecessary surgery.
Lessons Learned
Biopsies from the gastrointestinal tract that yield an appearance of a poorly differentiated carcinoma should preferably undergo immunohistochemistry.
Authors
Aravinth Anbarasu, MSa; Mahesh Subramania Iyer, MS, DNBa; Pushpa Mahadevan, MDb; Elezabeth Manuel, MDb; Vadavattath Padmanabhan Gangadharan, MD, DMc; Roy Mukkada, MD, DNBd; Hariharan Ramesh, MS, MCH, FACSa
Correspondence Author
Dr. Aravinth Anbarasu
Surgical Resident
Department of Surgical Gastroenterology
VPS Lakeshore Hospital
Nettoor, Maradu, Ernakulam
Kerala, India 682040
Email: ajuvinth@gmail.com
Author Affiliations
- Department of Surgical Gastroenterology
- Department of Pathology
- Department of Medical Oncology
- Department of Medical Gastroenterology
VPS Lakeshore Hospital, Cochin, Kerala, India
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Berman AT, Thukral AD, Hwang W-T, Solin LJ, Vapiwala N. Incidence and Patterns of Distant Metastases for Patients With Early-Stage Breast Cancer After Breast Conservation Treatment. Clin Breast Cancer. 2013;13(2):88-94. doi:10.1016/j.clbc.2012.11.001.
- Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175-180.
- Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic Breast Cancer to the Gastrointestinal Tract: Report of Five Cases and Review of the Literature. Int J Breast Cancer. 2012;2012:1-8. doi:10.1155/2012/439023.
- Li X, Feng Y, Wei W, et al. Signet-ring cell carcinoma of the breast: a case report. World J Surg Oncol. 2013;11(1):183. doi:10.1186/1477-7819-11-183.
- Chu PG, Weiss LM. Immunohistochemical Characterization of Signet-Ring Cell Carcinomas of the Stomach, Breast, and Colon. Am J Clin Pathol. 2004;121(6):884-892. doi:10.1309/A09E-RYMF-R64N-ERDW.
- Nazareno J, Taves D, Preiksaitis H-G. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol. 2006;12(38):6219-6224. doi:10.3748/wjg.v12.i38.6219.
- Switzer N, Lim A, Du L, Al-Sairafi R, Tonkin K, Schiller D. Case series of 21 patients with extrahepatic metastatic lobular breast carcinoma to the gastrointestinal tract. Cancer Treat Commun. 2015;3:37-43. doi:10.1016/j.ctrc.2014.11.006.
- Uygun K, Kocak Z, Altaner S, Cicin I, Tokatli F, Uzal C. Colonic metastasis from carcinoma of the breast that mimics a primary intestinal cancer. Yonsei Med J. 2006;47(4):578-582. doi:10.3349/ymj.2006.47.4.578.
- Ali W, Mohamed ZK, Thekkinkattil D. Colonic Metastasis from a Breast Carcinoma, an Unusual Colonoscopic Finding. Br J Med Pract BJMP.org BJMP. 2016;99(11).
- Duraiyan J, Govindarajan R, Kaliyappan K, Palanisamy M. Applications of immunohistochemistry. J Pharm Bioallied Sci. 2012;4(Suppl 2):S307-S309. doi:10.4103/0975-7406.100281.
- Mahmud N, Ford JM, Longacre TA, Parent R, Norton JA. Metastatic lobular breast carcinoma mimicking primary signet ring adenocarcinoma in a patient with a suspected CDH1 mutation. J Clin Oncol. 2015;33(4):e19-21. doi:10.1200/JCO.2013.49.1159.