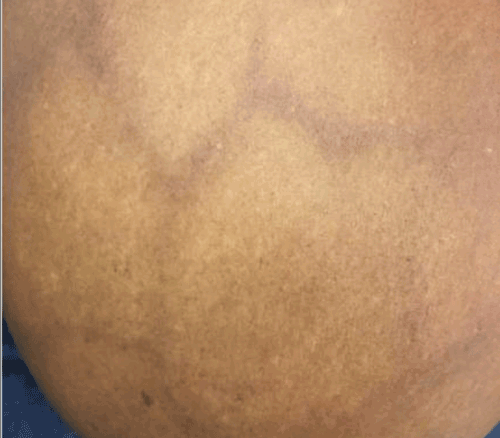
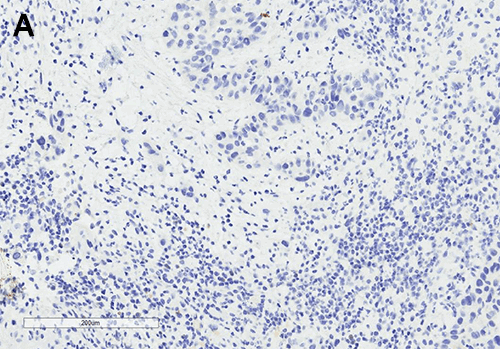
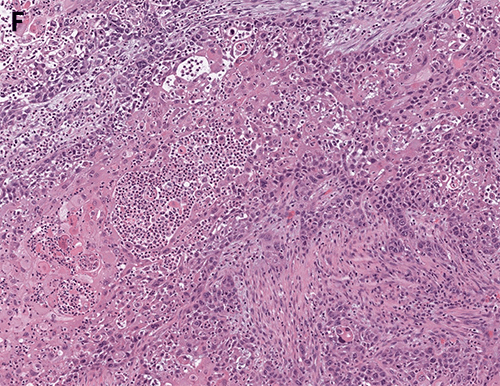
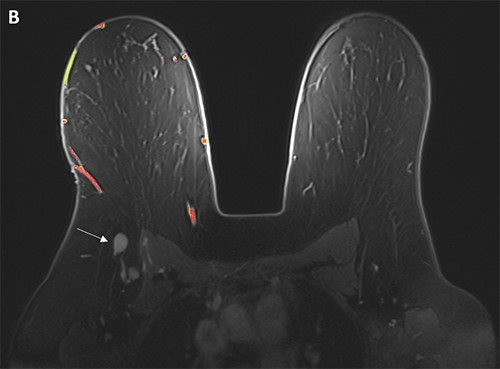
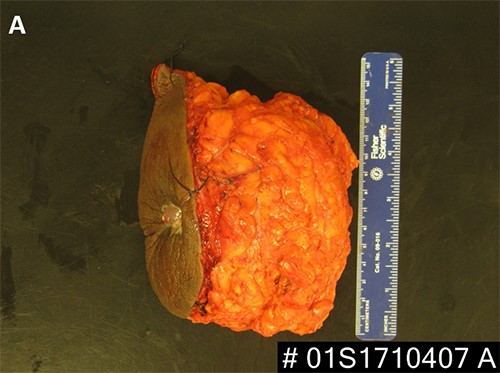
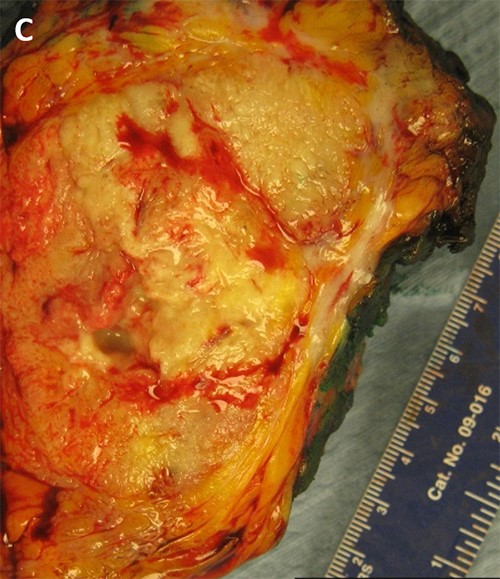
Figure 5. (A, B) Specimen with skin and associated abscess. (C) A crosssection of the specimen reveals a well-circumscribed lesion. Black ink denotes the margin.
Surgical pathology revealed a 9.5 cm tumor with negative margins, and one of two sentinel nodes had isolated tumor cells by immunohistochemistry. Based on the patient's stage and the current data suggesting that treatment for breast SCC be done in a manner similar to breast adenocarcinoma, our medical and radiation oncologists recommended adjuvant chemotherapy and adjuvant whole breast radiation.
In review of this case, a core needle biopsy of the patient's breast lesion prior to incision and drainage could have expedited the diagnosis had the radiologists been able to obtain tissue. Apart from this, the main obstacle that delayed her diagnosis was lack of insurance. Our breast center worked to connect the patient with resources so she could be seen as soon as they were aware of the patient, but the patient may have been diagnosed sooner had she been insured.
Discussion
Breast abscesses commonly affect women of reproductive age and are often a complication of mastitis.4 These can be treated with antibiotics, aspiration, and/or incision and drainage depending on the abscess size and characteristics.4-7 Needle aspiration is usually considered first-line treatment for breast abscesses less than 5 cm, and multiple aspirations may be required. However, surgical incision and drainage can be considered for first-line therapy in large (>5 cm), multiloculated, or recurrent abscesses. Also, if an abscess recurs after multiple aspiration attempts, surgical drainage may be needed. As in our case, if there is no known underlying etiology for abscess recurrence, tissue sampling for pathology during surgical incision and drainage of chronic or complicated abscesses should be done to rule out underlying malignancy or benign inflammatory condition.
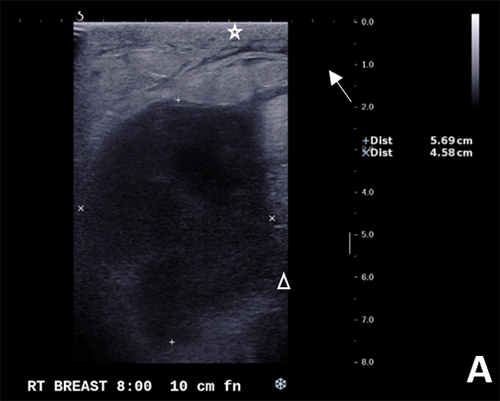
Although primary breast SCC is rare, based on the few reported cases, it is thought to be associated with chronic inflammation seen in benign breast diseases like abscesses, chronic cysts or sinuses, breast implants, or after radiation.3,8 It is hypothesized that SCC may arise from foci of squamous metaplasia within a preexisting adenocarcinoma or directly from mammary duct epithelium.2,5,8 To be diagnosed, primary SCC of the breast requires the tumor be free of other types of adenocarcinoma, not involve overlying skin, and not have another primary SCC.3,5,8 In general, primary breast SCCs are larger at time of presentation (>4 cm).1-3,5,8 Ultrasound may show a complicated cyst or inflammation, but otherwise primary breast SCCs do not have any known typical findings on mammogram.2,5,8 They are often hormone-receptor negative, as was the case in our patient, and some authors report that lymph node involvement is rare.1,5,8,9
Given the absence of specific management and treatment guidelines for primary breast SCC, management should be similar to that for breast adenocarcinoma.8-12 A surgical biopsy should be performed to establish the diagnosis, followed by clinical staging with imaging. Surgical intervention should be determined by clinical staging, often requiring partial mastectomy with sentinel lymph node biopsy to determine pathologic tumor staging. Adjuvant chemoradiation and hormone therapy should be used if indicated based on pathologic staging and receptor status.
Although prognosis of primary breast SCCs is controversial due to limited data, they are typically extremely aggressive tumors with courses comparable to poorly differentiated breast adenocarcinomas.1-3,5,8-12 Rates of locoregional relapse are high: one case series of 33 patients reported a median locoregional recurrence-free survival (RFS) of 20 months for patients without metastatic disease at diagnosis.8 In this study, 71 percent of these patients suffered relapse with median 14 month survival from the recognition of recurrent or metastatic disease. Their survival was somewhat worse than the still poor 64 percent overall five-year survival of patients in the SEER database. Due to the high rates of locoregional recurrence in this disease, early adjuvant radiation therapy is thought to be prudent despite reports of frequent recurrence in irradiated fields. Adjuvant chemotherapy is used regularly given the aggressive nature, but risk of distant metastasis remains high in SCC.8,9 Historically, anthracycline containing regimens have been the standard; however, the use of carboplatin and taxanes have biologic plausibility and have been employed.
Conclusion
Primary breast SCC is a rare and often aggressive cancer. In the absence of clear management guidelines for this tumor, medical and surgical management should follow guidelines for breast adenocarcinoma.
Lessons Learned
Given primary breast SCC can arise in the setting of benign chronic inflammatory conditions like breast abscess, it is important to maintain a high index of suspicion for an underlying inflammatory condition in patients who appear to have complicated cases of such benign diseases. The differential diagnosis for complicated breast abscess includes breast SCC as well as invasive carcinoma, granulomatous mastitis, and systematic lupus erythematosus. For this reason, any complex breasts lesions that requires surgical incision and drainage should be biopsied.
Authors
Bryce E. Haac, MDa; Rachel E. White, MDb; Nikki Tirada, MDc; Naomi Hardy, MDb; Yvonne Rasko, MDa; Elizabeth Nichols, MDd; Paula Rosenblatt, MDe; Sergio Dromi, MDc; Olga Ioffe, MDb; and Emily Bellavance, MDa
Correspondence Author
Dr. Bryce Haac
Department of Surgery
University of Maryland Medical Center
22 S. Greene St., 8th Floor
Baltimore, MD 212019
bhaac@som.umaryland.edu
Author Affiliations
- Department of Surgery
- Department of Anatomic Pathology
- Department of Diagnostic Radiology and Nuclear Medicine
- Department of Radiation Oncology
- Department of Hematology and Oncology
University of Maryland School of Medicine
Baltimore, MD
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Gupta C, Malani AK, Weigand RT, Rangineni G. Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract. 2006; 202(6):465-469.
- Flikweert ER, Hofstee M, Liem MSL. Squamous cell carcinoma of the breast: a case report. World J Surg Oncol. 2008; 6:135.
- Behranwala KA, Nasiri N, Abdullah N, Trott PA, Gui GPH. Squamous cell carcinoma of the breast: clinicopathologic implications and outcome. EJSO 2003;29:386-389.
- Kataria K, Srivastava A, Dhar A. Management of lactational mastitis and breast abscesses: review of current knowledge and practice. Indian J Surg. 2013 Dec;75(6):430-435.
- Seddik Y, Brahmi SA, Afqir S. Primary squamous cell carcinoma of the breast: a case report and review of literature. Pan Afr Med J. 2015;20:152.
- Lam E, Chan T, Wiseman SM. Breast abscess: evidence based management recommendations. Expert Rev Anti Infect Ther. 2014 Jul; 12(7): 753-762
- Barron AU, Luk S, Phelan HA, Williams BH. Do acute-care surgeons follow best practices for breast abscess management? A single-institution analysis of 325 consecutive cases. J Surg Res. 2017 Aug;216:169-171.
- Hennessy BT, Krishnamurthy S, Giordano S, Buchholz TA, Kau SW, Duan Z. Squamous cell carcinoma of the breast. J Clin Oncol. 2005 Nov 1;23(31):7827-7835.
- Behranwala KA, Nasiri N, Abdullah N, et al. Squamous cell carcinoma of the breast: Clinico-pathologic implications and outcome. Eur J Surg Oncol. 29:386,2003-389
- Cardoso F, Leal C, Meira A, et al: Squamous cell carcinoma of the breast. Breast. 9:315,2000-319
- Lafreniere R, Moskowitz LB, Ketcham AS: Pure squamous cell carcinoma of the breast. J Surg Oncol. 31:113,1986-119
- Badge SA, Gangane NM, Shivkumar VB, Sharma SM. Primary squamous cell carcinoma of the breast. Int J Appl Basic Med Res. 2014 Jan-Jun;4(1):53-55.