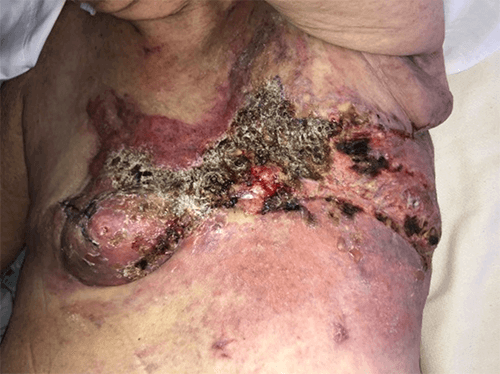
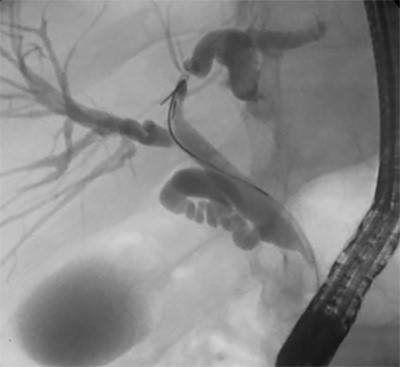
Figure 7. Recurrence at 11 weeks postoperatively
Discussion
Signet ring cell carcinomas (SRCCs) of the breast are extremely rare, especially in pure form.1,2 SRCCs can be associated with either lobular or ductal carcinoma. Prevalence of signet ring features varies, reported in 2 to 4.5 percent of all breast cancers.3 The first report of SRCC of the breast was described by Saphir in 1941.4 Currently, in the World Health Organization (WHO) Classification of Tumors of the Breast (fourth edition), SRCC is listed as an invasive breast carcinoma, but is not regarded as a tumor type of its own; rather, it can be observed with either invasive lobular carcinoma or with invasive carcinomas, no specific type (NST) as well as mucinous carcinomas.5 SRCC was previously classified as a distinct subset of mucinous carcinoma in the third edition.3 The American Joint Committee on Cancer (eight edition) does not list signet ring cell carcinoma as a histology code.6 Historically, SRCC was thought to be related specifically to lobular carcinoma.7 The reported cases of SRCC of the breast in the literature have varied in association with mucinous, lobular, ductal, and metaplastic carcinoma.
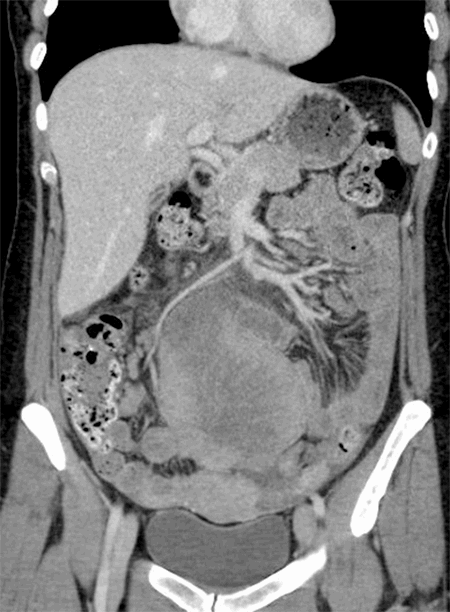
SRCC of the breast can be a primary or metastatic tumor. The high incidence of GI metastasis in patients with SRCC in a study by Merino et al7 posed a valid question: could the breast lesions be metastases from the GI tract? Differentiating between breast and gastric cancers may be difficult, but literature review indicates that each has distinct immunophenotype.8 In a case series study by Tot,9 immunohistochemical features of both primary invasive lobular breast carcinomas and gastrointestinal (GI) SRCCs were evaluated. In this study, all metastatic GI lesions were CK 20+/ER-. Primary SRCC of the breast is typically CK 7+/CK 20- while GI SRCCs are typically CK 20+/CK 7-.10,11 In the case report by Mahmud et al,8 use of immunohistochemical results correctly diagnosed a metastatic breast carcinoma of the stomach instead of a primary breast lesion. Recent publication by Hui et al12 supported the previous results by Tot: primary GI lesions with signet ring features exhibited CK 20 positivity while primary breast lesions with signet ring features exhibited ER positivity. E-cadherin positivity favors ductal origin of breast tumors, as seen in this case. While ER and GCDFP-15 are generally positive in primary SRCC of the breast, their negativity does not exclude breast origin.10 High-grade breast tumors with poor differentiation are less likely to be estrogen receptor positive, and triple negative breast tumors are less likely to be GCDFP-15 positive. In this case, ER/GCDFP-15 negativity did not rule out a breast primary, but CK 20 negativity discouraged gastrointestinal origin. Breast origin was further implied by a second, postoperative, PET-CT scan showing distant metastases to various nodal basins consistent with primary breast cancer, without presence of activity within any intraabdominal organs. Furthermore, while it would have been academically satisfying for the patient to undergo panendoscopy to confirm lack of gastrointestinal malignancy, she was never felt to be well enough to undergo such a procedure.
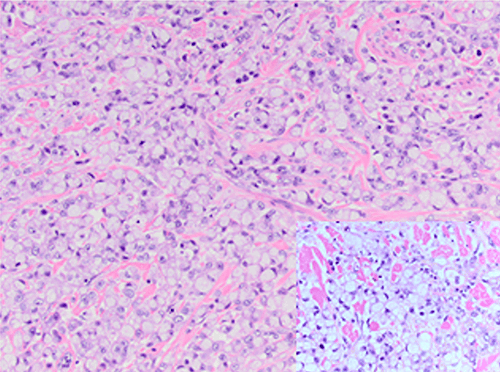
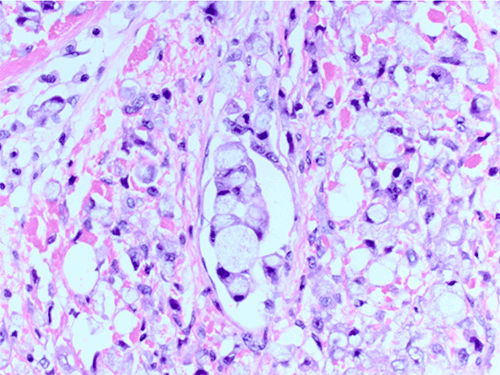
Signet cell appearance is due to large mucin filled vacuoles, displacing the nucleus to the periphery.2,3 When >20 percent signet ring cells are present, the carcinoma is labeled SRCC.7 Pure SRCC requires the majority of the neoplastic cells to show signet ring features.13
The prognosis for SRCC remains poor,14 with higher incidence and extent of lymph node involvement, especially in cases of ductal carcinoma with associated signet ring cells.1 SRCCs frequently metastasize to regional lymph nodes, peritoneal surfaces, ovaries, and lungs.11 In pure form, as in this report, SRCCs are more aggressive than mucinous, ductal, or lobular invasive carcinomas.13 Bilateral involvement is typically associated with lobular carcinoma,13 but in this report, the patient had two separate primary lesions, both ductal in origin. The hormonal receptors may carry a better prognosis for disease-free survival,15 but unfortunately this patient's left breast pathology was ER/PR/HER2-negative. SRCCs are more likely to demonstrate lymphovascular invasion,16 indicating a more aggressive behavior of these tumors, including high incidence of lymph node metastasis.14 Hull et al demonstrated an association of axillary lymph nodal involvement in cases of ductal carcinoma with associated signet ring cell carcinoma as well as a higher mortality rate.1 Because of this aggressive nature, some authors advocate SRCC as a subtype due to distinct clinical and pathologic features.1,7,17 Quantification of the percentage of SRCs could be useful in determining more aggressive behavior. Bartosch et al16 determined a higher SRC percentage correlated to a higher incidence of lymphovascular invasion and lymph node metastases.
Conclusion
SRCC of the breast remains a rare diagnosis. Due to its aggressive nature, it is important to report, regardless of histological subtype.16 Though it may originate from either lobular or ductal origin, classification as its own entity may be beneficial for future diagnosis and treatment,13 especially given the aggressive nature of this malignancy. Prognosis of SRCCs remains poor. Additionally, despite controversy over its classification, SRCC must be considered when differentiating between breast and GI primary lesions, with special attention to immunohistochemical features.
Lessons Learned
SRCC of the breast is a rare, aggressive tumor that must be differentiated from metastatic gastrointestinal cancer based on specific immunohistochemical features. Increased reporting of cases of SRCC of the breast is necessary to more clearly define tumor characteristics and behavior and guide effective treatment strategies for optimal outcomes.
Authors
Lauren Kopicky, DOa; Nida Shemmeri, MDb; Raj Gulati, MD, FACSa
Correspondence Author
Dr. Lauren Kopicky
Department of Surgery
600 Ivy Street, Suite 201
Elmira, NY 14905
KopickyL@gmail.com
Author Affiliations
- Arnot Ogden Medical Center
Department of Surgery
Elmira, NY 14905 - Arnot Ogden Medical Center
Department of Pathology
Elmira, NY 14905
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Hull MT, Seo IS, Battersby JS, Csicsko JF: Signet ring cell carcinoma of the breast: a clinicopathologic study of 24 cases. Am J Clin Pathol. 1980;73:31-35.
- Sandhu J, Dubey V, Makkar M, Suri V: Pure primary signet ring cell carcinoma of the breast: a rare cytological diagnosis. J Cytol. 2013;30(3):204-206.
- Karabagli P, Kilic H. Primary pure signet cell carcinoma of the breast: a case report and review of the literature. Breast Cancer. 2013;20:363-366.
- Saphir O: Mucinous carcinoma of the breast. Surg Gynecol Obstet. 1941;72:908-914.
- Sinn H, Krelpe H: A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care. 2013;8:149-154.
- AJCC Cancer Staging Manual, Eighth Edition, DOI 10.1007/978-3-319-40618-3_48
- Merino MJ, Livolsi VA. Signet ring carcinoma of the female breast: a clinicopathologic analysis of twenty-four cases. Cancer. 1981;48:1830-7.
- Mahmud N, Ford J, Longacre T, Parent R, Norton J: Metastatic Lobular Breast Carcinoma Mimicking Primary Signet Ring Adenocarcinoma in a Patient With a Suspected CDH1 Mutation. J Clin Oncol. 2015;33:4, e19-e21.
- Tot T: The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. APMIS 2000, 108:467-472.
- Li X, Feng YF, Wei WD, et al. Signet ring cell carcinoma of the breast: a case report. World J Surg Oncol. 2013;11:183.
- Chu PG, Wu E, Weiss LM: Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972.
- Hui Y, Wang Y, Nam G, Fanion J, Sturtevant A, Lombardo KA, and Resnick MB. Differentiating Breast Carcinoma with Signet ring Features from Gastrointestinal Signet ring Carcinoma: Assessment of Immunohistochemical Markers. Hum Pathol. 2018 Jan 6. pii: S0046-8177(18)30002-9. doi: 10.1016/j.humpath.2018.01.002. [Epub ahead of print]
- Liu SM, Chen DR: Signet ring cell carcinoma of the breast. Pathol Int. 2000, 50:67-70.
- Chatterjee D, Bal J, Das A, Kohli P, Singh G, Mittal B: Invasive Duct Carcinoma of the Breast with Dominant Signet ring Cell Differentiation: A Microsatellite Stable Tumor with Aggressive Behavior. Appl Immunohistochem Mol Morphol. 2017;25:10.
- Eltorky M, Hall JC, Osborne PT, el Zery F: Signet ring cell variant of invasive lobular carcinoma of the breast: a clinicopathologic of 11 cases. Arch Pathol Lab. 1994;118:245-248
- Bartosch C, Mendes N, Rios E, et al. Morphological features and mucin expression profile of breast carcinomas with signet ring cell differentiation. Pathol Res Pract. 2015;211:588-595.
- Frost AR, Terahata S, Yeh IT, Siegel RS, Overmoyer B, Silverberg SG: The significance of signet ring cells in infiltrating lobular carcinoma of the breast. Arch Pathol Lab Med. 1995;119:64-68.