Figure 4. Images captured at colonoscopy. A: Diverticuli within sigmoid colon, B: Half circumferential mass found in the cecum, multiple biopsies were taken of the lesion and a tattoo was applied. C: Sessile polyp found in the descending colon, polypectomy was performed with snare cautery. D: Large pedunculated polyp was found in the sigmoid colon E: Polypectomies were performed using snare cautery and a tattoo was applied. F: Additional large pedunculated polyp was found in the sigmoid colon, polypectomy was performed using snare cautery and the wound site was closed by placing hemoclips. G: Otherwise normal sigmoid colon mucosa. H: Normal retroflexion view within the rectum.
Colorectal surgery was consulted; however due to the patient's stage IV, TXN2M1 diagnosis, no colonic resection was indicated acutely. The patient's case was discussed at an interdisciplinary tumor board meeting, where it was agreed that, should her cecal mass become obstructing, or her iron deficiency anemia recur, surgical resection or palliative stenting would be discussed at that point in time.
To address the inevitable obstructive hydrocephalus and persistence of the patient's neurological symptoms, stereotactic computer-assisted (navigational) suboccipital craniotomy for gross total resection of the left cerebellar mass, and placement of an external ventricular drain, was performed by neurosurgery. Pathologic frozen sectioning and immunohistochemical analysis of the excised tumor demonstrated metastatic colorectal cancer (tumor cells positive for CK20 and CDX2, negative for CK7, TTF1, GCDFP15, GATA3 and mammaglobin, supporting the aforementioned diagnosis). The patient progressed well during her hospital course and was safely discharged to home on postoperative day five.
The patient was referred to radiation oncology and hematology/oncology for further medical management of her brain lesion and colon cancer (stage IV, TXN2M1), which included radiotherapy, and FOLFOX/Avastin combination chemotherapy, respectively.
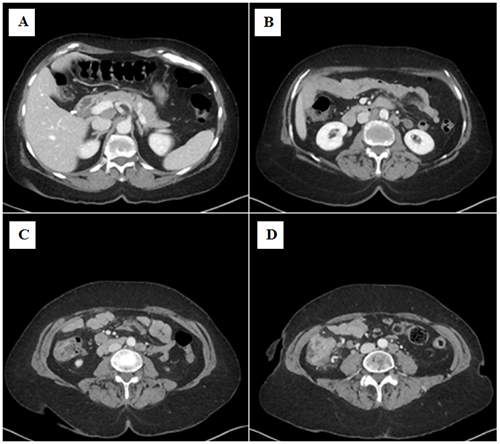
The patient is currently seven months out from initial diagnosis and continuing to carry out activities of daily living independently. She recently completed her seventh of 12 cycles of chemotherapy. Surveillance CT has since revealed a marked decrease in the size of the cecal mass and complete resolution of the intraabdominal lymphadenopathy, with no new metastatic lesions noted within the chest, abdomen, or pelvis. Repeat CT head or MRI brain to monitor for metastatic recurrence has yet to occur.
Discussion
Colorectal cancer (CRC) is the third most common cancer in both men and women, and it is the second-leading cause of death in both genders in the United States.1,2 In 2017, an estimated 135,430 new cases of CRC were diagnosed, contributing to 8 percent of all new cancer diagnoses in the United States.3 Of these, 21 percent were found to have distant metastasis upon initial diagnosis.3,4 Sites of metastatic disease are most frequently the lung (10–20 percent) and liver (20–30 percent).5,6 Brain metastases from CRC are rare, with an estimated incidence of 0.6 to 4 percent.7,8 Brain metastases are a sign of late-stage metastatic disease, with 70 and 40 percent of patients having lung and liver metastases, respectively, at diagnosis of brain metastasis.9 Additionally, postmortem studies have demonstrated that at the time of death, between 2 and 3 percent of patients who die from CRC harbor occult brain metastases.5,6
The cerebellum is the involved site in 55 percent of metastatic CRC cases to the brain.5,6, 10 It is hypothesized that pelvic or abdominal primary malignancies are more likely to metastasize specifically to the posterior fossa due to the Batson vertebral venous plexus.8 Regardless, the prognosis for these patients is dismal, with a reported median survival of less than three months.11
Upon our review of current literature, a small number of cases of solitary cerebellar metastasis have been reported; however the vast majority involved the distal colon as the primary site.12 We hypothesize that differences in vascular anatomy of the proximal versus distal colon result in a higher incidence overall of brain metastases from rectosigmoid primary lesions.13 Those cases that report more proximal colon cancers are limited to the ascending colon or hepatic flexure; to our knowledge, our case of a cecal carcinoma to present with a solitary cerebellar metastasis is indeed a rarity.14,15
Further, the discovery of CRC as heralded by brain metastasis is a rare but reported event, as most patients present with symptoms of mass effect (such as altered mental status or focal weakness), in addition to marked iron deficient anemia.12,14,16 Unique to our case, the patient presented with an isolated and subtle neurologic symptoms and only a distant history of iron deficient anemia (she was not found to be anemic on exam), for which she had been taking oral iron supplements.
Overall, the prognosis of metastatic CRC to the brain is exceedingly poor. By far, the intervention that prolongs life expectancy significantly is surgical resection of the metastasis, ranging from 6 to 10 months.5,17,18 Adjuvant whole brain radiation therapy (WBRT) has been shown to substantially improve survival, as opposed to either surgery or radiation alone.13 However, it must be kept in mind that this survival benefit is obscured by the fact that patients undergoing these treatments are, of course, healthier, and fit to undergo such a rigorous course of treatment.
The patient presented here underwent both surgical resection and WBRT, and continues to do remarkably well seven months from her diagnosis.
Conclusion
Cerebellar metastases from CRC are exceedingly rare, with the vast majority of these cases occurring from distal rectosigmoid primary lesions. Here, we present a case of cecal adenocarcinoma with a solitary distant metastasis to the cerebellum, presenting as headache and subjective gait disturbance. Our case demonstrates an unconventional presentation of CRC, thereby resulting in a seemingly retrograde diagnosis of the primary tumor (i.e., from brain metastasis to primary neoplasm). This highlights the importance of communication between various medical and surgical specialties to arrive at the correct diagnosis and optimize the patient's treatment course.
Lessons Learned
The importance of multidisciplinary collaboration when treating metastatic CRC is invaluable and cannot be understated, particularly in the case of disease metastasizing to the brain. Furthermore, aggressive surgical resection and adjuvant WBRT of isolated metastases can significantly impact the survival of patients with metastatic CRC.
Authors
Samantha Knight, MD
Southern Illinois University School of Medicine, Department of Surgery, Division of General Surgery, Springfield, IL
Adam Lipson, MD
Southern Illinois University School of Medicine, Department of Surgery, Division of Neurosurgery, Springfield, IL
Mary Brandt, BA
Southern Illinois University School of Medicine, Springfield IL
Jeffrey W. Cozzens, MD, FACS, FAANS
Southern Illinois University School of Medicine, Department of Surgery, Division of Neurosurgery, Springfield, IL
Elizabeth Dawn Wietfeldt, MD, FACS, FASCRS
Southern Illinois University School of Medicine, Department of Surgery, Division of General Surgery, Springfield, IL
Springfield Clinic, Department of Colon & Rectal Surgery, Springfield IL
Correspondence Author
Dr. Samantha W.E. Knight
701 N. 1st Street
Division of Surgery
General Surgery Residency Program
Southern Illinois University School of Medicine
PO Box 19638
Springfield, IL 62794-9638
Phone: 217-414-0221
Email: sknight55@siumed.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Contributors Statement
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Meeting Presentation
Illinois Chapter of the American College of Surgeons
References
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. [revised 2018 May]. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017 June.
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009 Nov;22(4):191–7.
- Howlader N, Noone A, Krapcho M, Miller D, Bishop K, et al. SEER Cancer Statistics Review, 1975–2014. In: SEER Database [Internet]. Bethesda, MD: National Cancer Institute; 2017 April.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7–30.
- Wroński M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999 Apr 15;85(8):1677–85.
- Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011 Aug 15;117(16):3630–40.
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004 Jul 15;22(14):2865–72.
- Alden TD, Gianino JW, Saclarides TJ. Brain metastases from colorectal cancer. Dis Colon Rectum. 1996 May;39(5):541–5.
- Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer-Incidence and patient characteristics. BMC Cancer. 2016 Apr 1;16:260.
- Cascino TL, Leavengood JM, Kemeny N, Posner JB. Brain metastases from colon cancer. J Neurooncol. 1983;1(3):203–9.
- Tan WS, Ho KS, Eu KW. Brain metastases in colorectal cancers. World J Surg. 2009 Apr;33(4):817–21.
- DeWitt J, Lippman HR, Wassef W. Solitary cerebellar metastasis as a presentation for colon cancer: a case report and review of the literature. Dig Dis Sci. 2001 Jun;46(6):1163–6.
- Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012 Oct;19(5):254–8.
- Garg PK, Bohidar NP, Sharma MP, et al. Isolated cerebellar metastasis from carcinoma of the colon. Postgrad Med J. 1999 Feb;75(880):119–20.
- Kimura M, Tanishima H, Tatsubayashi T, et al. A Case with Solitary Brain Metastatic Tumor from Colon Cancer. Gan To Kagaku Ryoho. 2017 Nov;44(12):1388–1390.
- Chu C, Navalkissoor S, Gillmore R. Neurological symptoms from a brain metastasis as the first presentation of colorectal cancer. BMJ Case Rep. 2015 Sep 22;2015
- Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology. 2009;76(5):369–74.
- Hammoud MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ. Colorectal carcinoma and brain metastases: distribution, treatment and survival. Ann Surg Oncol. 1996;3:453–63.