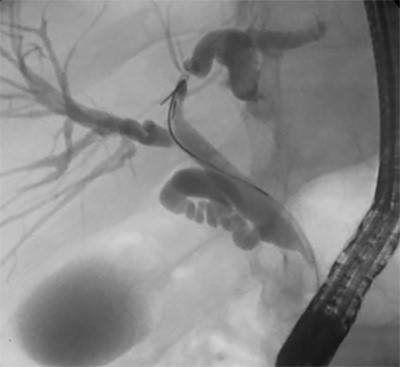
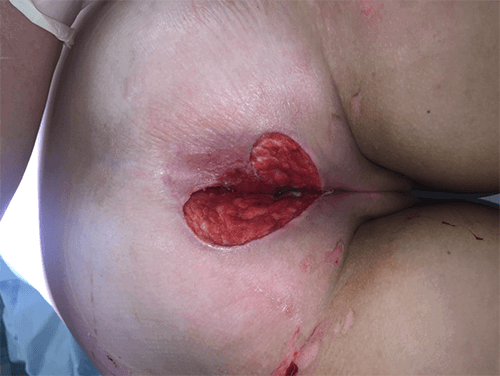
Figure 3. Intraoperative images showing the exophytic mass identified and stapled along the greater curvature of the stomach
The branches of the gastroepiploic within the greater omentum were dissected off the greater curvature with a harmonic scalpel to normal tissue both above and below the mass. Three 60 mm endoscopic linear staple loads with staple line reinforcement were used to perform a wedge resection around the mass. An endoscopy was performed with a negative leak test to confirm there was no significant gastric intraluminal narrowing. The patient was admitted postoperatively and started on a soft diet. He was discharged home on postoperative day two. At his two week clinic follow-up, he was recovering well, denying any significant heartburn or early satiety. Pathology grossly described a 5.5 x 3.5 x 3.0 cm yellow-tan mass involving the submucosa and muscularis propria with a negative margin of resection. Sections showed a low-grade spindle cell proliferation with immunohistochemical stain all consistent with GS.
Discussion
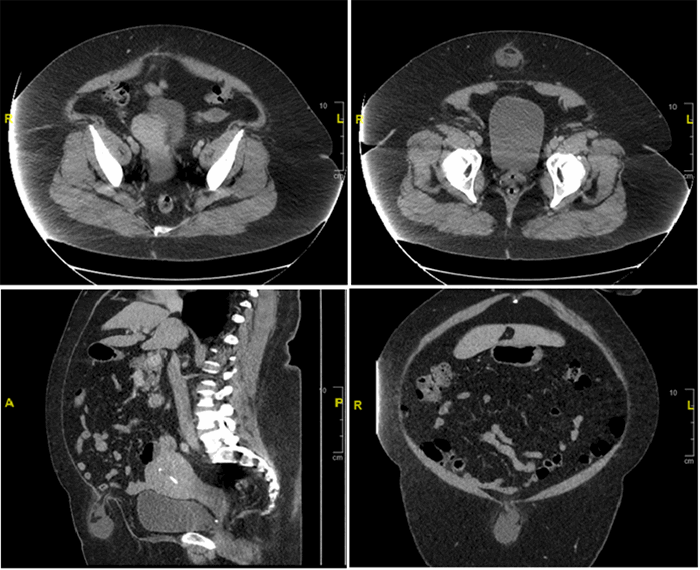
Schwannomas of the gastrointestinal tract are rare; when discovered, they are most commonly found in the stomach.3,7 If associated with other findings, such as vestibular schwannomas, cutaneous schwannomas, multiple spinal tumors, or first-degree relatives with the disease, patients with gastrointestinal tract schwannomas should be assessed for neurofibromatosis.8 The clinical presentation of GS varies: reported complaints include epigastric pain, gastrointestinal bleeding, and chest pain as well as asymptomatic, incidentally discovered masses.9 Workup of a mass concerning for GS typically involves CT imaging and endoscopic ultrasound with biopsy. These masses have been described as ovoid, well-defined, and of exophytic or mixed-growth, with homogenous progressive enhancement on CT that is commonly mistaken for GIST preoperatively.10 This homogeneity is one possible distinguishing mechanism from GISTs, which typically have hemorrhage, cystic changes, and necrosis also present.11
Histologically, GSs are described as bundles of spindles cells woven within loose myxoid areas that align in their orientation.1 GSs can be accurately distinguished from GIST based on their immunohistochemical profile after endoscopic biopsy or resection; however, if GIST is suspected and the tumor is deemed resectable, pre-resection biopsy is not recommended due to risk of hemorrhage;12 GS stain S100 and GFAP positive indicating evidence of nerve sheath differentiation, but CD117, CD34, desmin, and smooth muscle actin negative. GISTs stain S100 negative but CD117, DOG-1, and CD34 positive.7,9
Surgical resection of GS portends an excellent prognosis as these tumors are almost always benign.13 Resection is viewed as the current choice of treatment, and postoperatively distinguishing these masses from GISTs histochemically is important, particularly due to their similarity in preoperative presentation and the risk of recurrence in GISTs. Resection options include endoscopic, laparoscopic, hybrid, and open removal techniques.14 Endoscopic removal has been described for smaller non-exophytic tumors, and laparoscopic resection has been demonstrated to be safe and effective for tumors up to 10 cm.14,15 In the setting of being unable to rule out GIST, open removal should also be considered in cases where manipulation of the specimen would risk rupturing or seeding the tumor.14
Conclusion
GSs are rare, but should be removed—even if asymptomatic. These masses, if in an appropriate location, can be amenable to laparoscopic partial gastrectomy. Their benign nature portends an excellent prognosis.
Lessons Learned
GSs are discovered with a variety of clinical presentations and should be properly distinguished from the more common gastrointestinal stromal tumor. The mainstay of treatment is local resection with negative margins.
Authors
Matthew Madion, MD
Medical College of Wisconsin
Department of Surgery
Division of Minimally Invasive General Surgery
Milwaukee, WI
Rana M Higgins, MD
Medical College of Wisconsin
Department of Surgery
Division of Minimally Invasive General Surgery
Milwaukee, WI
Correspondence Author
Dr. Rana M Higgins
Division of General Surgery
8701 W Watertown Plan Road
Milwaukee, Wisconsin 53226
Phone: (414) 955-1764
Email: rhiggins@mcw.edu
Disclosure Statement
Dr. Higgins has disclosed the following conflicts of interest:
- Speaker for W.L. Gore & Associates
- Proctor for Intuitive Surgical
References
- Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: A clinicopathologic and immunohistochemical study. Human Pathol. 1988;19(3):257–264.
- Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293–296.
- Aaloten LA, Hamilton SR. Pathology and genetics of tumours of the digestive system. World Health Organization Classification of Tumours. Lyon: IARC Press; 2000:37–38.
- DeWitt J, Emerson RE, Sherman S, et al. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc. 2010;25(7):2192–2202.
- Steigen SE, Eide TJ. Gastrointestinal stromal tumors (GISTs): a review. APMIS. 2009;117(2):73–86.
- Hou YY, Tan YS, Xu JF, et al. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48(5):536–545.
- Tao K, Chang W, Zhao E, et al. Clinicopathologic Features of Gastric Schwannoma. Medicine. 2015;94(45):e1970.
- Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974–1986.
- Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Human Pathol. 2012;43(5):650–659.
- Ji J, Lu C, Mao W, Wang Z, Xu M. Gastric schwannoma: CT findings and clinicopathologic correlation. Abdom Imaging. 2014;40(5):1164–1169.
- Levy A., Quiles AM, Miettinen M, and Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. Am J Roentenol. 2005;184:797–802.
- Arolfo S. Gastrointestinal stromal tumors: Thirty years experience of an Institution. World J Gastroenterol. 2011;17(14):1836.
- Williamson J, Wadley M, Shepherd N, Dwerryhouse S. Gastric schwannoma: a benign tumour often mistaken clinically, radiologically and histopathologically for a gastrointestinal stromal tumour – a case series. Ann R Coll Surg Engl. 2012;94(4):245–249.
- Lee CM. Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol. 2014;20(36):13035.
- Otani Y, Ohgami M, Igarashi N, et al. Laparoscopic wedge resection of gastric submucosal tumors. Surg Laparosc Endosc Percutan Tech. 2000 Feb;10(1):19–23.