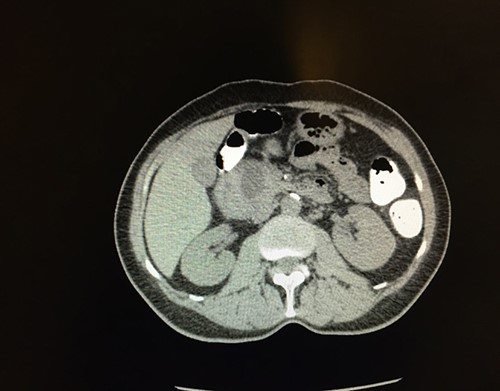
Figure 1. CT scan of the abdomen demonstrating enlargement of the pancreatic head, which contains a 2.4 x 1.7 cm mass.
To further examine the unequivocal appearance of the periampullary mass seen on the CT scan, endoscopic ultrasonography was ordered. An abnormal hypoechoic area was noted in the region between the duodenal wall and head of the pancreas, measuring 3.5 x 3.5 cm. Fine-needle aspiration was performed that yielded initially clear fluid, but subsequently became cloudy. The fluid was sent for pathological and cytological examination. It was determined to consist of acellular material containing polarizable and negatively birefringent needle-shaped crystals, compatible with urate crystals and consistent with gouty deposits. Surrounding areas of acute inflammatory cells, histiocytes, and benign duodenal-type epithelium were also noted. Immunologic stains of the fluid for CD45, panCK, synaptophysin, chromogranin and Ki67 were all negative. Serum CEA was 1.1 ng/mL (reference range: 0–2.5 ng/mL) and serum CA19-9 was 29 U/mL (reference range: <37 U/mL).
The patient was seen three weeks later for follow-up in the outpatient clinic. She denied any episodes of abdominal pain or bloody stool since discharge. She was subsequently scheduled for repeat abdominal imaging one month later; however she did not comply with follow-up, and the studies were not completed.
Discussion
This paper presents the case of a patient who presented with melena associated with a confirmed periampullary mass upon imaging studies. Masses in this region carry a high suspicion for malignancy of either the ampulla of Vater, main pancreatic duct, or the duodenum. Most commonly included in the differential diagnoses are pancreatic ductal adenocarcinoma, distal cholangiocarcinoma, ampullary adenocarcinoma, and duodenal adenocarcinoma. Mainstay of treatment is typically surgical resection by means of a pancreaticoduodenectomy (Whipple operation).1 Other consideration for a periampullary mass may be cystic or inflammatory in nature, such as pancreatic pseudocyst, peripancreatic fluid collection, or walled-off pancreatic necrosis. Relevant treatment includes percutaneous drainage and subsequent analysis of fluid components.2 Thus, in a patient with gastrointestinal bleeding and an appreciable abdominal mass on imaging, it is important for clinicians to perform a comprehensive workup to arrive at a proper diagnosis and, if possible, rule out malignancy.
Gout is a condition arising from increased serum or tissue uric acid levels, either as a result of overproduction or diminished clearance. These elevated levels of uric acid in tissue spaces cause precipitation of monosodium urate crystals in joints, most commonly the first toe, leading to severe inflammation and pain. The kidney remains another usual location for crystal deposition, leading to nephrolithiasis and renal insufficiency. The disease is known to follow a typical progression beginning with asymptomatic hyperuricemia, followed by recurrent acute attacks, and finally escalating to chronic joint disease with tophus formation.8 Diagnosis is established by examination of aspirated joint or tissue fluid and identification of negatively birefringent crystals.3 However, gouty tophi have been reported to manifest in other, less common sites throughout the body, such as the lumbar spine, penile shaft, cornea, and cardiac valves. Due to the historical rarity of such occurrences, malignancy is often an early suspicion; thus the option for subsequent surgical intervention must be explored.4-7 These atypical presentations generally do not obey expected timelines for gout progression. As such, the tophus may be the initial manifestation of disease, specifically in females and the elderly.8 Estrogen facilitates excretion of uric acid by the kidneys, making menopause a credible risk factor.9 Furthermore, the onset of gout in women is shown to be nearly seven years later than in men.9,10
To our knowledge, there have only been three published cases detailing tophi in the pancreas. Interestingly, two of these were incidental findings in previously healthy patients,11,12 and the other was diagnosed in a patient with known history of pseudocysts.13 Awareness of this rare occurrence may help eliminate unwarranted surgical exploration and instead direct appropriate medical management.
Conclusion
We present the case of a female with melena associated with a periampullary mass confirmed as pancreatic gout. This atypical presentation of gout should be identified in order to exclude a diagnosis of gastrointestinal malignancy and eliminate any further unnecessary intervention.
Lessons Learned
This paper reiterates the possibility for monosodium urate deposition in exceptional places throughout the body and encourages such a diagnosis be considered in a patient without a significant past medical history of gout.
Authors
Nikhil R. Shah, BA
St. George's University, School of Medicine, St. George, Grenada
Newark Beth Israel Medical Center, Department of Surgery, Newark, NJ
William Boyan Jr., MD
Newark Beth Israel Medical Center, Department of Surgery, Newark, NJ
Kevin Clarke, MD
Newark Beth Israel Medical Center, Department of Surgery, Newark, NJ
Correspondence Author
Nikhil Shah
146 Sun Valley Way
Morris Plains, NJ 07950
Phone: 973-270-8371
Email: nshah8@sgu.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Cameron J, Cameron A. The Management of Periampullary Cancer. In: Cameron J, Cameron A, eds. Current Surgical Therapy. 12th ed. Philadelphia, PA: Elsevier; 2017. 538–542.
- Cameron J, Cameron A. In: Cameron J, Cameron A, eds. Current Surgical Therapy. 12th ed. Philadelphia, PA: Elsevier; 2017. 5168–523.
- George C, Minter D. Hyperuricemia. In: StatPearls. Treasure Island, FL: StatPearls Publishing. 2017. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459218/. Accessed December 4, 2018.
- Hasturk A, Basmaci M, Canbay S, Vural C, Erten F. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J. 2012;21(4): S4008–403.
- Martin JF, Alonso F, Sanz I, Campos R, Gutierrez MA, Olmo JM. Gouty Tophi in the Penis: A Case Report and Review of the Literature. Case Rep Urol. 2012;2012:594905.
- Yazdanyar A, Rizzuti AE, Mechel E, Denisova K, Lazzaro DR. Gout Keratitis: A Case of Peripheral Ulcerative Keratitis Secondary to Gout with A Review of the Literature. Cornea. 2018;37(3):3798–381.
- Rohani A, Chamanian S, Hosseinzade P, Ramezani J. A Case of Mitral Valve Tophus in a Patient with Severe Gout Tophaceous Arthritis. J Clin Imaging Sci. 2012;2:68.
- Forbess L, Fields T. The Broad Spectrum of Urate Crystal Deposition: Unusual Presentations of Gouty Tophi. Semin Arthritis Rheum. 2012;42(2):1468–54.
- Stamp LK, Jordan S. The challenges of gout management in the elderly. Drugs Aging 2011;28(8):5918–603.
- De Leonardis F, Govoni M, Colina M, Bruschi M, Trotta F. Elderly-onset gout: A review. Rheumatol Int. 2007;28(1):18–6.
- Gupta S, McMahan Z, Patel P, Markham D, Drazner M, Mammen P. Pancreatic gout masquerading as pancreatic cancer in a heart transplant candidate. J Heart Lung Transplant. 2009;28(10):11128–3.
- Koh H, Low HC, Seet JE, Chua WY. Pancreatic gout and the role of multimodal imaging in its management. BMJ Case Rep. 2016; pii: bcr2016215449.
- Khanna D, Tang SJ, Wallace WD, Roth BE, Hahn BH. Gouty tophi in a pancreatic pseudocyst. Arthritis Rheum. 2002;46(2):5658–6.