Abstract
Background
A 59-year-old woman with strong family history of early-age colorectal cancer was found to have synchronous tubular adenomas of the duodenum and transverse colon during surveillance endoscopy 12 years after undergoing right colectomy and adjuvant chemotherapy for stage II colon adenocarcinoma. The duodenal lesion was endoscopically unresectable due to central depression, and the transverse colon adenoma was unresectable because it was confluent with the previous ileocolic anastomosis. Given the synchronous unresectable lesions in the setting of an Amsterdam positive kindred, the patient underwent simultaneous pancreaticoduodenectomy and completion total abdominal colectomy with ileorectal anastomosis. Histopathologic analysis of the specimens revealed T4N0 poorly differentiated MLH1 deficient duodenal adenocarcinoma with pancreatic invasion and tubular adenoma of the colon with high grade dysplasia. Following adjuvant chemotherapy, there is no evidence of recurrent cancer after two years of surveillance.
Summary
While the crude overall risk for small bowel and periampullary tumors remains low, clinicians must maintain awareness of a relatively increased risk of extracolonic tumors in Lynch syndrome (LS) patients.
Conclusion
LS patients have an increased risk for developing small bowel cancer (SBC) when compared to the general population. However, given the low incidence of these tumors and uncertain efficacy of contemporary screening modalities, surveillance of the small bowel has not been recommended. The current case report exemplifies the challenges associated with waiting for patients to develop symptoms to develop before investigating for SBC.
Key Words
Lynch syndrome, MLH1 deficiency, hereditary nonpolyposis colorectal cancer syndrome (HNPCC), duodenal adenocarcinoma, periampullary adenocarcinoma, pancreaticoduodenectomy
Case Description
A 59-year-old woman with a strong family history of colorectal cancer underwent a right hemicolectomy with ileocolic anastomosis for T3N0M0 colon adenocarcinoma. At the time of her colectomy, it was known that her father and paternal grandfather had been diagnosed with colon cancer in their third and second decade of life, respectively. She had previously undergone hysterectomy with bilateral salpingo-oophorectomy for unspecified endometrial dysplasia at age 38. She was diagnosed with hereditary nonpolyposis colorectal cancer syndrome (HNPCC) via Amsterdam criteria, but a formal genetic evaluation and diagnosis was not made at the time. The patient completed a four-cycle course of adjuvant 5-fluorouracil and leucovorin and was surveyed with imaging for the first five postoperative years and endoscopy every 1–2 years. Twelve years after her colectomy, surveillance colonoscopy revealed an approximately 4 cm sessile colonic polyp that was confluent with the ileocolic anastomosis (Figure 1).
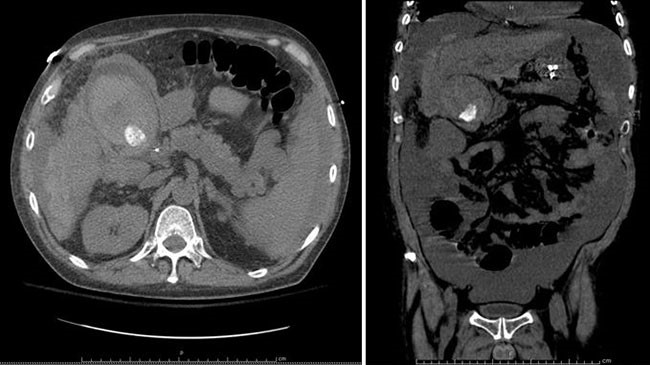
Surveillance esophagogastroduodenoscopy (EGD) noted a new 8 mm nodule with central depression immediately proximal to the major ampulla (Figure 2).
Biopsies of the colonic and duodenal lesions revealed tubular adenomas without high grade dysplasia. Upon referral to an advanced interventional endoscopist, the colonic lesion was felt to be endoscopically unresectable due to involvement of the ileocolic anastomosis, while the duodenal lesion was unresectable due to worrisome central depression and periampullary proximity. Pre-operative staging abdominopelvic magnetic resonance imaging (MRI) and serum carcinoembryonic antigen (CEA) levels were unremarkable.
The patient was symptom-free from both lesions and in a good state of health. Following extensive discussions between the patient and her care teams, synchronous completion total abdominal colectomy with ileorectal anastomosis and pancreaticoduodenectomy were performed. The patient’s postoperative course was complicated by a pancreatic leak requiring image-guided percutaneous drain placement. Following drain placement she convalesced and was discharged home on postoperative day 13. The percutaneous drain was eventually removed as an outpatient on postoperative day 28.
Histopathologic analysis of the pancreaticoduodenectomy specimen revealed a 1.5 cm T4 moderately to poorly differentiated adenocarcinoma with pancreatic and peripancreatic fat invasion and all 9 lymph nodes were negative for metastases (Figure 3). The total abdominal colon specimen revealed a 3 x 3 cm tubular adenoma with high-grade dysplasia. All surgical margins were widely negative.
Immunohistochemistry (IHC) analysis of DNA mismatch repair genes (MMR) revealed MLH1 deficiency with wild-type BRAF confirming the genetic basis of Lynch syndrome (LS). Given the findings of T4 duodenal adenocarcinoma, the patient completed a six-month course of adjuvant FOLFOX at the recommendation of a multi-disciplinary management conference. Eventual germline sequencing confirmed MLH1 deficiency for the patient and her family.
The patient is currently undergoing serial annual imaging, upper and lower endoscopy, and CEA surveillance. The patient denies difficulty ingesting solids and liquids and typically has three to four loose bowel movements daily without fecal incontinence. She consumes pancreatic enzyme supplements. Endoscopic, imaging, and CEA surveillance to date has revealed no evidence of disease recurrence or metachronous neoplasia at two years since surgery.
Discussion
LS accounts for 2 to 4 percent of colorectal cancers and is the most common hereditary colorectal cancer syndrome.1 LS arises from an autosomal dominant germline mutation in one of the DNA MMR genes: MLH1 (50 percent), MSH2 (40 percent), MSH6 (7 to 10 percent), PMS2 (<5 percent); or the EPCAM gene (1 to 3 percent).2 The term hereditary nonpolyposis colorectal cancer (HNPCC) historically referred to patients meeting Amsterdam I or II criteria, while the term Lynch syndrome refers to those with a specifically identified genetic defect. Though variation is predicated upon the specific genetic defect, LS conveys a 10 to 74 percent lifetime risk of developing colorectal adenocarcinoma as well as endometrial cancer (14 to 71 percent), gastric cancer (0.2 to 13 percent), ovarian cancer (4 to 20 percent), hepatobiliary cancers (0.02 to 4 percent), urinary tract cancer (0.2 to 25 percent), SBC (0.4 to 12 percent), brain and central nervous system cancers (1 to 4 percent), sebaceous neoplasms (1 to 9 percent), and pancreatic cancer (0.4 to 4 percent).3 IHC tumor testing, which can be performed on both preoperative endoscopic biopsies, surgical resection specimens, and even pre-malignant adenomas, clarifies if the tumor arises from MMR deficiency, while blood-based testing confirms a germline mutation in the suspected patient and kindred. Intensive surveillance programs have been advocated for LS patients following diagnosis given the high rates of associated cancers.
Colonoscopy is currently recommended starting at age 20–25 (or two to five years before the earliest diagnosed cancer in the patient’s kindred) and performed in one- to two-year intervals thereafter for patients with LS.1,3 The most frequent extracolonic manifestation of LS is endometrial cancer and guidelines for endometrial surveillance call for endometrial biopsy and transvaginal ultrasound annually, starting at age 30–35.3 While surveillance regimens for Lynch-related colorectal cancers is well established, the efficacy of screening for extracolonic malignancies is controversial, likely arising from relatively low and variable rates of incidence. The benefit of screening for less common extracolonic LS malignancies, such as SBC, remains unclear.4,5 One review of 210 cases of LS found four cases of SBC (2 percent) representing a 100-fold relative risk when compared to the general population.6 However, a second review of 360 cases found no cases of SBC, questioning the role for screening.7 A further review of the literature shows the relative risk for SBC in patients with Lynch syndrome has been reported to range from 25 to 291, while the lifetime cumulative risk appears to range from 1 to 4 percent.8-10 This lifetime risk represents a greater than 100 fold increased risk when compared to the general population.
Age is a risk factor for LS-related SBC with an increase in risk starting at age 40 and increasing 10 fold by the age of 60. Lynch syndrome patients usually present 10 years earlier with SBC, when compared to those without Lynch syndrome. Moreover, SBC may be the initial manifestation of Lynch syndrome estimated 30 to 70 percent of patients.10 A review of several case series, totaling 199 cases, found that 43 percent of SBC developed in the duodenum, while 37 percent were in the jejunum and 20 percent in the ileum.11 This distribution mirrors SBC in the general population, where the duodenum is the most common site of tumor development.12 Since SBC is typically located in the duodenum or ileum, a 2013 consensus conference of European LS experts suggested the distal duodenum and terminal ileum be evaluated when endoscopy is performed for LS patients, although the group specifically recommended against dedicated small bowel and gastric endoscopy for the sole purpose of surveillance.6 The European recommendations vary from an American guideline calling for “consideration” of upper endoscopic surveillance beginning at age 30–35 and repeating every two to three years based on patient risk factors.3
The patient presented in this case report was surveyed annually with lower and upper endoscopies following her initial surgery. Fortunately, her duodenal tumor was within reach of the endoscopy, allowing for diagnosis. Following the diagnosis of synchronous duodenal and colonic lesions, the decision was made to proceed with a combined pancreaticoduodenectomy and completion total abdominal colectomy. A staged procedure may have delayed resection of potential malignancy as well hindered a second stage operation with reoperative adhesions. Few cases of combined pancreaticoduodenectomy and completion total abdominal colectomy for LS have been reported in the literature since most patients present with metachronous SBC.13
Given the paucity and inconsistent data surrounding LS associated SBC, the benefits and ideal modality of surveillance is uncertain. Accordingly, current societal surveillance recommendations encourage providers to “consider” upper endoscopy screening for gastric and proximal small bowel adenocarcinoma in LS but do not offer steadfast surveillance recommendations. The development of new imaging and endoscopy modalities may make evaluation of the small bowel feasible for this patient population. Video capsule endoscopy (VCE) appears to be helpful in the diagnosis of SBC in both the general population and in LS patients.14-17 One study comparing VCE to CT enteroclysis, found that of 35 asymptomatic LS patients, three (9 percent) had SBC while computed tomography (CT) missed two of the three cancers. All three cases were diagnosed by VCE while CT enteroclysis missed two cases.18 While VCE appears promising, further studies are needed to delineate sensitivity, specificity, and cost effectiveness of screening programs. Until the overall efficacy of small bowel screening is clarified, surveillance for SBC in LS should continue to be centered on patients who develop symptoms concerning for small bowel pathology
Conclusion
LS patients have an increased risk for developing SBC when compared to the general population. However, given the low incidence of these tumors and uncertain efficacy of contemporary screening modalities, surveillance of the small bowel has not been recommended. The current case report exemplifies the challenges associated with waiting for patients to develop symptoms to develop before investigating for SBC.
Lessons Learned
Practitioners should maintain suspicion for extracolonic malignancies in patients with LS.
Authors
Tarik K. Yuce, MD
Northwestern Memorial Hospital
Chicago IL
Michael F. McGee, MD FACS, FASCRS
Northwestern Memorial Hospital
Chicago IL
Correspondence
Michael F. McGee, MD, FACS, FASCRS
Northwestern Memorial Hospital
251 East Huron Street
Chicago IL 60611
Phone: 312-695-2534
Fax: 312-695-4955
E-mail: mmcgee1@nm.org
Disclosures
The authors have no conflicts of interest to disclose.
References
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High Risk Assessment: Colorectal Version 3.2017. 2017 Oct 10; National Comprehensive Cancer Network.
- Kohlmann W, Gruber SB. Lynch Syndrome. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993-2018.
- Giardiello F, Allen J, Axilbund J, et al. Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the US multi-society task force on colorectal cancer. Gastroenterol. 2014;147:502–526.
- Baichi M, Arifuddin R, Mantry P. Metachronous Small bowel Adenocarcinomas Detected by Capsule Endoscopy in a Patient with hereditary Nonpolyposis Colorectal Cancer. Dig Dis Sci. 2007;52:1134–1136.
- Benatti P, Roncucci L, Percesepe A, et al. Small Bowel Carcinoma in Hereditary Nonpolyposis Colorectal Cancer. Am J Gastroenterol. 1998;93:2219–2222.
- Vasen H, Wijnen J, Menko F, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020–1027.
- Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of dna-mismatch-repair genes. Int J Cancer. 81:214–218.
- Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97–104.
- Watson P, Lynch H. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993; 71:677–685.
- Shenoy S. Genetic risks and familial associations of small bowel carcinoma. World J Gastrointest Oncol. 2016;8:509–519.
- Koornstra J. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol. 2012;26:359–368.
- Yagyu T, Aihara T, Murayama M, et al. Mucinous Carcinoma of the Duodenum Associated with Hereditary Nonpolyposis Colorectal Cancer: Report of Case. Surg Today. 2006;36:1129–1132.
- Rodriguez-Bigas M, Vasen H, Lynch H, et al. Characteristics of small bowel carcinoma in hereditary nonpolyposis colorectal cacner. Cancer. 1998;83:240–244.
- Schwartz G, Barkin J. Small-bowel tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026–1030.
- Bailey A, Debinski H, Appleyard M, et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J of Gastroenterol 2006;101:2237–2243.
- Marmo R, Rotondano G, Riccio F, et al. Small-bowel adenocarcinoma diagnosed via capsule endoscopy in a patient found to have hereditary nonpolyposis colorectal cancer. Gastrointest Endosc. 2007;65:524–525.
- Haanstra J, Al-toma A, Dekker E, et al. Prevalence of small-bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Gut. 2015;64:1578–1583.
- Saurin J, Pilleul F, Soussan E, et al. Small-bowel capsule endoscopy diagnoses early and advanced neoplasms in asymptomatic patients with Lynch syndrome. Endoscopy. 2010;42:1057–1062.