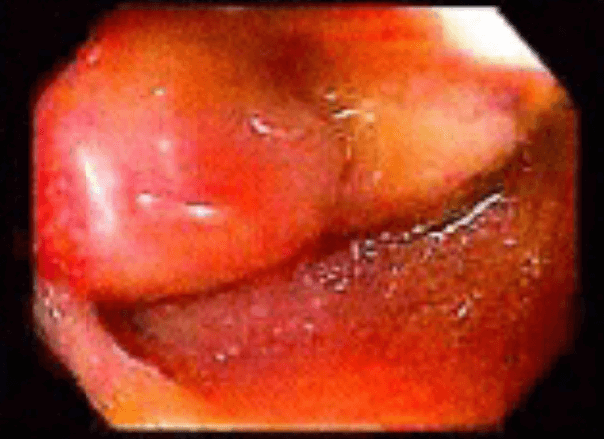
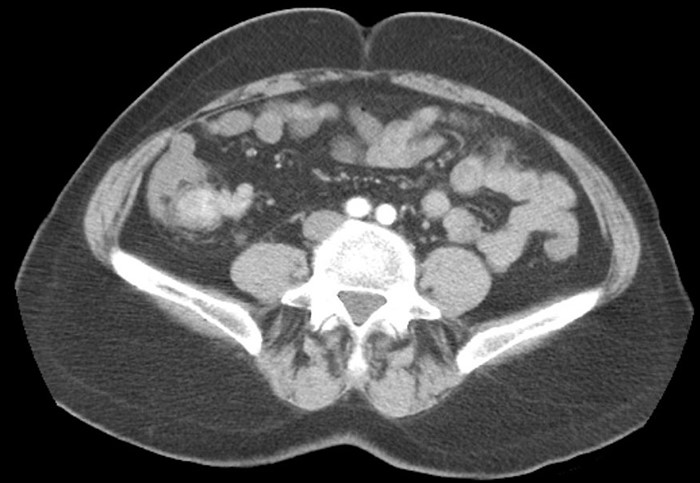
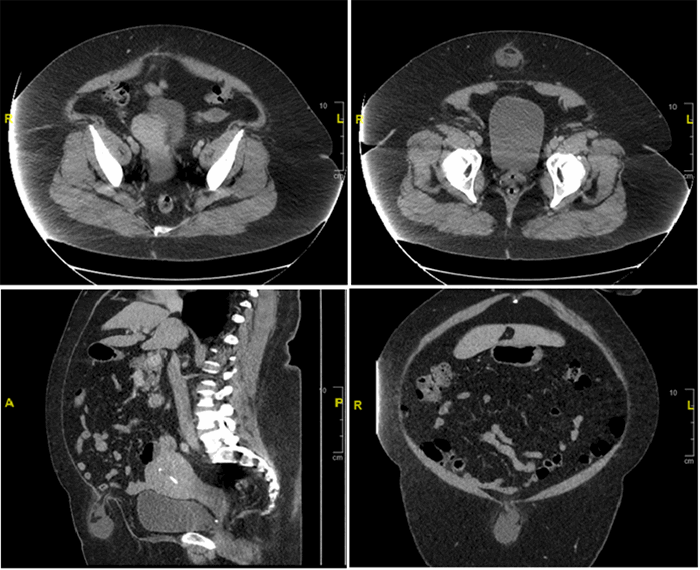
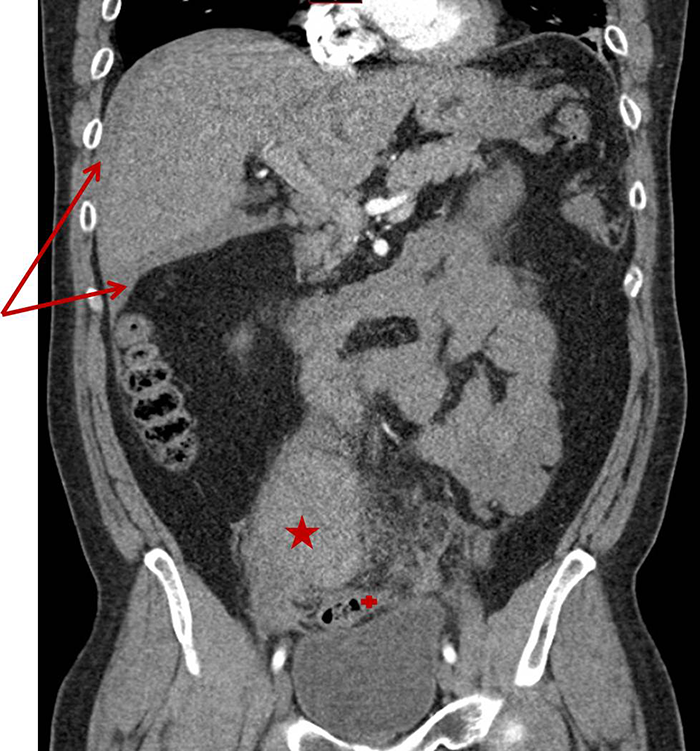
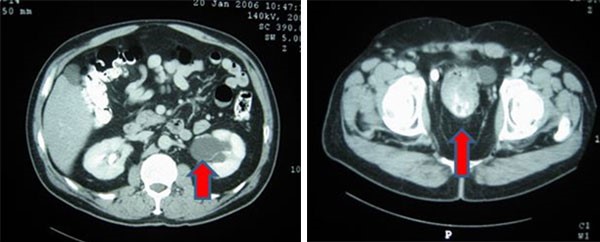
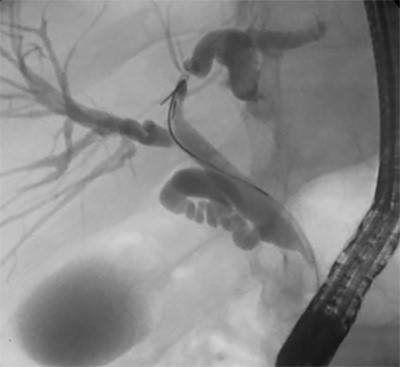
Figure 3. Preoperative CT abdomen demonstrating enhancing terminal ileum carcinoid tumor with surrounding ileocolic adenopathy
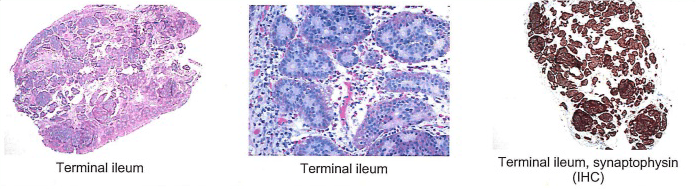
Final pathology demonstrated a 3.1cm well-differentiated neuroendocrine tumor with extensive lymphovascular and perineural invasion and 11 out of 36 positive lymph nodes, some with extracapsular extension, and five mesenteric deposits, largest at 1.2cm in size. Final pathology demonstrated a stage III G1pT3pN1cM0 neuroendocrine tumor of the small bowel. The patient had a normal postoperative course and was discharged on postoperative day four. She was seen by medical oncology (specializing in only neuroendocrine tumors (NET)) who did not recommend adjuvant treatment, but did order follow up with 68-Gallium (Ga) DOTATATE Positron Emission Tomography (PET)/CT) every four months for one year. She has no evidence of disease at nine months postoperatively.
Discussion
The ileum is the most common location of small bowel NET.1 Overall, the 5 and 10-year cancer-specific survivals for small bowel NET are 95.0% and 88.5%, but age >50, tumor >2cm, higher tumor grade, advanced T stage, and lack of surgical management are predictors of poorer prognosis.1 Small bowel NETs typically present at advanced stages with partial obstruction, pain, bleeding, or, when they become metastatic and cause carcinoid syndrome.2,3
Furthermore, NETs frequently metastasize early to lymph nodes or to distant organs, even with small primary tumors.4 In the setting of presentation with carcinoid syndrome, symptoms are managed with somatostatin analogues and selected use of vasopressors when carcinoid crisis occurs.5 While laparoscopic approaches to colon cancer are now known to be equivalent to laparotomy, the gold standard treatment for NET is an open abdominal operation with local lymph node resection in order to perform a complete evaluation of the small bowel and mesentery.5 A minimally invasive approach is not recommended because of the risk of missing small lesions and multifocal disease which is common in small bowel NET. "Early" NETs that are ≤1cm, grade 1-2, do not invade the muscular layer, and are accessible by endoscopy, may undergo endoscopic resection, although these favorable lesions have rarely been described in the ileum perhaps in part due to the lack of routine colonoscopic surveillance of the ileum.6
When suspected, nonfunctional NETs should be worked up with tumor markers including chromogranin-A, neuron-specific enolase, pancreatic polypeptide, and urinary 5-Hydroxyindoleacetic acid (HIAA). Imaging with contrast enhanced CT or magnetic resonance imaging (MRI) is recommended for diagnosis, staging, and follow up. Because of the expression of somatostatin receptors by the tumors, functional imaging with 68-Ga-DOTATATE PET/CT can be a useful tool, especially in localizing a primary tumors and metastasis.5 Despite available imaging techniques, preoperative imaging techniques tend to underestimate extent of disease and direct intraoperative examination is the most reliable staging method.7,8
The role of double balloon enteroscopy for diagnosis of NET is limited.9 Furthermore, video capsule endoscopy has also been known to have poor yield in detecting primary lesions.5 Detection of NET by terminal ileum intubation on colonoscopy tends to be low yield, however its value during routine colonoscopy may be underestimated. Although ileoscopy is the gold standard of a complete colonoscopy, there is great variation in practice of ileal intubation largely secondary to perceived technical difficulty: a recent study showed ileoscopy rates as low as 18% on routine colonoscopy.10 0.3% of all patients receiving ileoscopy have clinically significant histopathology in the ileum, and this number increases to 1.8% in patients complaining of right lower quadrant abdominal pain.11 This case highlights the importance of complete colonoscopy including intubation of the ileocecal valve with inspection of the terminal ileum, especially considering this maneuver adds on average only three minutes to the evaluation and can easily be achieved in up to 85% of cases.10,12 Furthermore, in addition to potentially pathologically significant findings in the terminal ileum, there are three additional advantages: first, it assures the endoscopist that they really did reach the cecum. Second, it is good practice for both the resident and staff so that they have the skill set needed when there is a compelling reason to enter the terminal ileum. Finally, routine terminal ileum intubation makes the endoscopist familiar with the normal anatomy of the terminal ileum (including frequent lymphoid hyperplasia).
Conclusion
Small bowel NET typically presents at advanced stage and may be understaged by preoperative imaging studies. Surgical resection remains the standard of care. Complete screening colonoscopy including terminal ileum intubation is achievable in the majority of patients and may be an important in earlier detection of ileal NETs.
Authors
Catherine H Davis, MD, MPH
Department of Surgery
Houston Methodist Hospital
Houston, TX
Jose C Muniz Castro, MD
Department of Surgery
The University of Texas Health Science Center at Houston
Houston, TX
Barbara L Bass, MD, FACS
Department of Surgery
Houston Methodist Hospital
Houston, TX
H. Randolph Bailey, MD, FACS
Department of Surgery
Houston Methodist Hospital
Houston, TX
Department of Surgery
The University of Texas Health Science Center at Houston
Houston, TX
Correspondence Author
H. Randolph Bailey, MD, FACS
The University of Texas Health Science Center at Houston
Houston Methodist Hospital
6550 Fannin St.
Suite 2307
Houston, TX 77030
(713) 486-4613
Fax: (713)795-5737
hrbailey@swbell.net
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Wu L, Fu J, Wan L, et al. Survival outcomes and surgical intervention of small intestinal neuroendocrine tumors: a population based retrospective study. Oncotarget. 2017;8(3):4935-4947.
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-3072.
- Selberherr A, Niederle MB, Niederle B. Surgical Treatment of Small Intestinal Neuroendocrine Tumors G1/G2. Visc Med. 2017;33(5):340-343.
- Farley HA, Pommier RF. Surgical Treatment of Small Bowel Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30(1):49-61.
- Howe JR, Cardona K, Fraker DL, et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715-731.
- Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3(7):133-139.
- Chambers AJ, Pasieka JL, Dixon E, Rorstad O. Role of imaging in the preoperative staging of small bowel neuroendocrine tumors. J Am Coll Surg. 2010;211(5):620-627.
- Clift AK, Faiz O, Al-Nahhas A, et al. Role of Staging in Patients with Small Intestinal Neuroendocrine Tumours. J Gastrointest Surg. 2016;20(1):180-188; discussion 188.
- Bellutti M, Fry LC, Schmitt J, et al. Detection of neuroendocrine tumors of the small bowel by double balloon enteroscopy. Dig Dis Sci. 2009;54(5):1050-1058.
- Harewood GC, Mattek NC, Holub JL, Peters D, Lieberman DA. Variation in practice of ileal intubation among diverse endoscopy settings: results from a national endoscopic database. Aliment Pharmacol Ther. 2005;22(6):571-578.
- Jeong SH, Lee KJ, Kim YB, Kwon HC, Sin SJ, Chung JY. Diagnostic value of terminal ileum intubation during colonoscopy. J Gastroenterol Hepatol. 2008;23(1):51-55.
- Cherian S, Singh P. Is routine ileoscopy useful? An observational study of procedure times, diagnostic yield, and learning curve. Am J Gastroenterol. 2004;99(12):2324-2329.