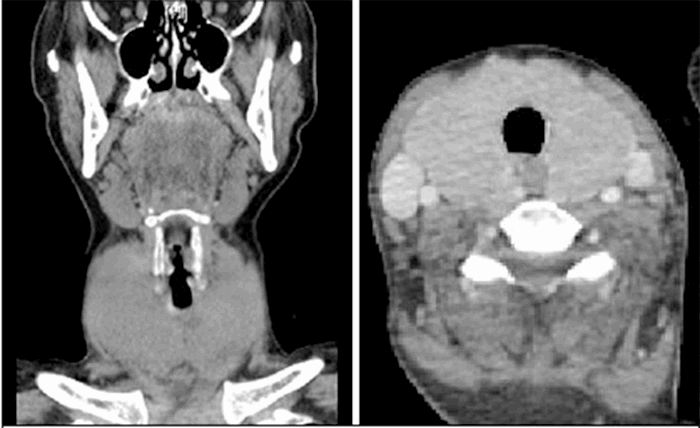
After completing the scans, the patient was returned to the trauma bay, where she began to acutely decompensate. She developed respiratory failure requiring intubation, which proved difficult due to the compression of her airway from a large thyroid gland. Three attempts were made prior to successful intubation. Simultaneously, the patient became febrile to 102° and developed worsening tachycardia with a heart rate greater than 200 and accelerated hypertension with systolic blood pressures of 220 mmHg. Given the size of the patient’s thyroid on CT scan and the constellation of symptoms, she was presumed to be in acute thyroid storm. She was given propranolol and admitted to the medical intensive care unit. Propylthiouracil, propranolol, hydrocortisone, and potassium iodide drops were all administered. TSH was initially <0.003 (ref 0.35–5.0), free T4 was 4.09 (ref 0.7–1.25), and free T3 greater than 30.0 (ref 1.7–3.7). She was subsequently found to have an anti-thyroglobulin level of 300 (ref <116), thyroperoxidase antibodies greater than 1000 (ref <51), and ultrasound with diffuse enlargement with hyper-vascularity consistent with Graves’ disease. Several days into her hospital stay, due to a national shortage of PTU, she was switched to methimazole to control hyperthyroidism. She continued to develop worsening pancytopenia, transaminitis, and increasing free T3 and T4 levels. On post-trauma day nine, the patient underwent total thyroidectomy with lymphadenectomy for control of thyrotoxicosis. Pathology was consistent with Graves’ disease and no malignancy. Postoperatively the patient was extubated and progressed well. She was weaned from hydrocortisone, Methimazole and Lugol’s solution were discontinued, and levothyroxine was started. Once extubated, she endorsed weight loss and jitters for approximately one year before her trauma but was never diagnosed with hyperthyroidism. The patient recovered well with normal T4 by posttrauma day fourteen, was discharged home and continued to do well at her follow up appointment two weeks later.
Discussion
The incidence of thyrotoxicosis is estimated to be 0.2 per 100,000 hospitalized patients per year, with a 10 to 30 percent mortality rate. Thyrotoxicosis typically develops in patients older than 50 with longstanding goiters from either Graves’ disease or toxic multinodular goiter, with symptoms more severe in patients with Graves’ disease.5 Prevalence is increased in women and after exposure to medications or radiocontrast dye containing iodine.1-5
CT scans utilizing iodinated contrast have become commonplace during the workup traumatic injuries. 200 cc of contrast contains approximately 7000 μg of free iodide, 45 times the recommended daily dose.1 The Wolff-Chiakoff effect, first described in 1948, which allowed an inhibitory effect of excess iodine with a transient decrease in thyroid hormone synthesis and resumed normal thyroid function in 24 to 48 hours, allows a normally functioning thyroid to account for this excess iodine without much physiologic effect on the patient.1,6,7 Absence of this normal response to excess iodine can result in transient or permanent hyperthyroidism or even thyrotoxicosis known as the Jod-Basedown phenomenon. First described in 1821, this phenomenon is most commonly associated with Graves’ disease and thyroid autonomy such as nodular or nontoxic diffuse goiter.6,8 Iodine-induced thyrotoxicosis was seen in 13 of 60 elderly subjects in Australia and Germany after contrast radiography, and thyroid scan revealed multinodular goiter in these patients.9,10 These preexisting thyroid diseases predisposed patients to iodine-induced hyperthyroidism from other sources of iodide as well. One study in Zaire found 7.4 percent of 190 patients with nodular goiter developed thyrotoxicosis after iodized salt distribution, a case series from Boston showed four of eight of patients with goiter developed severe thyrotoxicosis after administration of iodide daily for several weeks, and overt Graves’ disease increased from 10.4 to 20.9 cases per 100,000 in Austria after salt iodination in 1993.11-13
Hyperthyroidism after iodinated contrast dye can also be seen without preexisting thyroid disease but is more likely to be subclinical.14 Rhee et al. identified that patients without known thyroid disease from two tertiary United States hospitals between 1990 to 2010 had a two-fold increased risk of developing hyperthyroidism after contrast exposure (OR 1.98, 95 percent CI 1.08–3.06).15 A Turkish study of 101 patients without thyroid disease found a 6 percent incidence of subclinical hyperthyroidism after iodine exposure during coronary angiography.16
The diagnosis of thyroid storm is clinical, and the American Thyroid Association (ATA) has provided guidelines for management.17 In addition to thyroid function tests including TSH, free T4 and T3, the ATA recommend adjunct use of a clinical scoring system, the Burch Wartofsky Point Scale (BWPS). First proposed in 1993, diagnostic criteria include hyperpyrexia, tachycardia, arrhythmias, congestive heart failure, agitation, delirium, psychosis, stupor, coma, nausea, vomiting, diarrhea, hepatic failure, and presence of an identified precipitant.17,18 Patients with a score > 45 with systemic decompensation require aggressive therapy.18 Treatment is multimodal. Medications include beta-adrenergic blockade to control the symptoms induced by increased adrenergic tone, antithyroid (ATD) therapy to block new hormone synthesis and prevent conversion to active T3, inorganic iodide to block new synthesis and the release of hormones, and corticosteroid therapy to reduce conversion to active T3 as well as promote vasomotor stability, reduce the autoimmune process in Graves’ disease, and treat associated relative adrenal insufficiency. In comparing ATDs, Prophylthiouracil (PTU) is preferred to methimazole (MMI) in the emergent or intensive care setting due to scheduled dosing every four hours and superior suppression of conversion to T3 (45 percent versus 10 to 15 percent decrease in levels of T3 in 24 hours of use). Methimazole has a longer duration of action and is less hepatotoxic, therefore preferred in the outpatient setting.19 In pregnancy, PTU is preferred in the first trimester and is transitioned to MMI after this.19 However, in one Japanese study comparing PTU to MMI, no difference was seen in mortality or disease severity..19 In addition to medications, supportive care consists of cooling with acetaminophen and cooling blankets, volume resuscitation, nutritional support, respiratory care, and monitoring in an intensive care unit (Table 1).17 Treatment of iodine-induced hyperthyroidism specifically includes avoiding additional iodine and administration of beta-blockers alone or with ATDs depending on severity. A saturated solution of potassium iodide (SSKI) must be used with caution. Treatment with SSKI should be initiated only after ATD and beta-blockade. Timing of SSKI should also be coordinated with definitive thyroidectomy to avoid escape phenomena with prolonged treatment.17
Thyroidectomy is the definitive treatment in patients with persistent symptoms. Patients are at higher risk for thyrotoxic crisis during the operation and ideally are optimized to the euthyroid state with preoperative ATD with or without beta-adrenergic blockade.17 However, if the patient is not tolerating medical management due to side effects such as pancytopenia and transaminitis as in this case, thyroidectomy before achieving euthyroid state may be required.
|
Decrease peripheral action of T3 by decreasing conversion of T4 → T3
|
|
Antithyroid Drugs (ATDs)
- Propylthiouracil (PTU)
- Alternate: Methimazole (MMI)
|
Also, block new hormone synthesis
Decrease levels of T3 by 45 percent in 24 hours
Decrease levels of T3 by 10 to 15 percent in 24 hours
|
|
Beta-Adrenergic Blocker
- Propanolol
- Alternate: esmolol drip
|
Inhibits type 1 deiodinase, HR management
|
|
Glucocorticoids
- Hydrocortisone
- Alternate: dexamethasone
|
Also, prophylaxis against adrenal insufficiency
|
|
Decrease thyroid hormone secretion (T3 and T4)
|
|
Inorganic iodide
- Saturated solution KI
- Alternate: Lugol’s solution
|
Also, blocks new hormone synthesis
Start >1 hour after ATD
|
|
Supportive Care
|
- Treat or avoid precipitating event
- Cooling with acetaminophen, cooling blankets
- Volume resuscitation and nutritional support
- Monitor in the intensive care unit
|
|
Definitive Treatment
|
- Achieve euthyroid state
- Surgery
|
Table 1. Medical Management of Acute Thyrotoxicosis
Conclusion
In summary, iodide administration, whether from iodinated contrast or dietary administration, can result in clinically significant thyrotoxicosis in patients with underlying thyroid disease. Therefore, prior to administration of iodide, it is important to consider risk factors such as preexisting thyroid disease and ask questions that might elicit symptoms of previously undiagnosed conditions. This was relevant in this case, as the patient noted a one-year history of weight loss and anxiety but did not mention this at the time of initial trauma assessment.
Trauma patients frequently are exposed to contrasted CT scans in the typical workup, and in many instances, a detailed patient history is unable to be obtained due to urgent or emergent status. However, iodinated contrast media use is not without consequence, and it is imperative to understand that hyperthyroidism or thyrotoxicosis can occur especially in patients with preexisting thyroid disease and the elderly. Therefore, it is of utmost importance to have a high index of suspicion in patients with known risk factors or those eliciting an atypical physiologic response after contrast administration.
Lessons Learned
Frugality in ordering scans with contrast and identifying at-risk patients by asking pertinent questions in the initial trauma assessment might prevent consequential thyrotoxicosis cases. It is also essential to maintain a high level of suspicion of this condition in any patient displaying an atypical response to contrast administration.
Authors
Warner RL; Musgrove KA; LoPinto M; Borgstrom DC
Author Affiliation
West Virginia University School of Medicine, Department of Surgery, Morgantown, WV 26505
Corresponding Author
David C. Borgstrom, MD, FACS
Department of Surgery
1 Stadium Drive
Morgantown, WV 26505
Phone: (304) 598-4890
E-mail: dcborgstrom@hsc.wvu.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- van der Molen, Aart J et al. “Effect of iodinated contrast media on thyroid function in adults.” European radiology vol. 14,5 (2004): 902-7. doi:10.1007/s00330-004-2238-z
- Akamizu, Takashi et al. “Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys.” Thyroid : official journal of the American Thyroid Association vol. 22,7 (2012): 661-79. doi:10.1089/thy.2011.0334
- Swee, Du Soon et al. “Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis.” Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists vol. 21,2 (2015): 182-9. doi:10.4158/EP14023.OR
- Angell, Trevor E et al. “Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study.” The Journal of clinical endocrinology and metabolism vol. 100,2 (2015): 451-9. doi:10.1210/jc.2014-2850
- Nadkarni, Priya J., and Burman D. Kenneth. “Thyrotoxicosis.” Acute Endocrinology:: From Cause to Consequence (Contemporary Endocrinology), edited by Van Greet Den Berghe, Softcover reprint of hardcover 1st ed. 2008, Humana, 2010, pp. 1–28.
- Lee, Sun Y et al. “A review: Radiographic iodinated contrast media-induced thyroid dysfunction.” The Journal of clinical endocrinology and metabolism vol. 100,2 (2015): 376-83. doi:10.1210/jc.2014-3292
- Wolff, J, and I L Chaikoff. “Plasma inorganic iodide as a homeostatic regulator of thyroid function.” The Journal of biological chemistry vol. 174,2 (1948): 555-64.
- Leung, Angela M, and Lewis E Braverman. “Iodine-induced thyroid dysfunction.” Current opinion in endocrinology, diabetes, and obesity vol. 19,5 (2012): 414-9. doi:10.1097/MED.0b013e3283565bb2
- Martin, F I et al. “Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly.” The American journal of medicine vol. 95,1 (1993): 78-82. doi:10.1016/0002-9343(93)90235-h
- Martin, F I, and D R Deam. “Hyperthyroidism in elderly hospitalised patients. Clinical features and treatment outcomes.” The Medical journal of Australia vol. 164,4 (1996): 200-3.
- Bourdoux, P P et al. “Iodine-induced thyrotoxicosis in Kivu, Zaire.” Lancet (London, England) vol. 347,9000 (1996): 552-3. doi:10.1016/s0140-6736(96)91188-5
- Mostbeck, A et al. “The incidence of hyperthyroidism in Austria from 1987 to 1995 before and after an increase in salt iodization in 1990.” European journal of nuclear medicine vol. 25,4 (1998): 367-74. doi:10.1007/s002590050234
- Vagenakis, A G et al. “Iodide-induced thyrotoxicosis in Boston.” The New England journal of medicine vol. 287,11 (1972): 523-7. doi:10.1056/NEJM197209142871101
- Roti, E, and E D Uberti. “Iodine excess and hyperthyroidism.” Thyroid : official journal of the American Thyroid Association vol. 11,5 (2001): 493-500. doi:10.1089/105072501300176453
- Rhee, Connie M et al. “Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism.” Archives of internal medicine vol. 172,2 (2012): 153-9. doi:10.1001/archinternmed.2011.677
- Özkan, Sevil et al. “Thyroid functions after contrast agent administration for coronary angiography: a prospective observational study in euthyroid patients.” Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology vol. 13,4 (2013): 363-9. doi:10.5152/akd.2013.134
- Ross, Douglas S et al. “2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis.” Thyroid : official journal of the American Thyroid Association vol. 26,10 (2016): 1343-1421. doi:10.1089/thy.2016.0229
- Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Encocrinol Metab Clin North Am. 1993;22:263-277.
- Abuid, J, and P R Larsen. “Triiodothyronine and thyroxine in hyperthyroidism. Comparison of the acute changes during therapy with antithyroid agents.” The Journal of clinical investigation vol. 54,1 (1974): 201-8. doi:10.1172/JCI107744
- Isozaki, Osamu et al. “Treatment and management of thyroid storm: analysis of the nationwide surveys: The taskforce committee of the Japan Thyroid Association and Japan Endocrine Society for the establishment of diagnostic criteria and nationwide surveys for thyroid storm.” Clinical endocrinology vol. 84,6 (2016): 912-8. doi:10.1111/cen.12949