Five months after the procedure, the patient was doing well, without any complications related to the port removal.
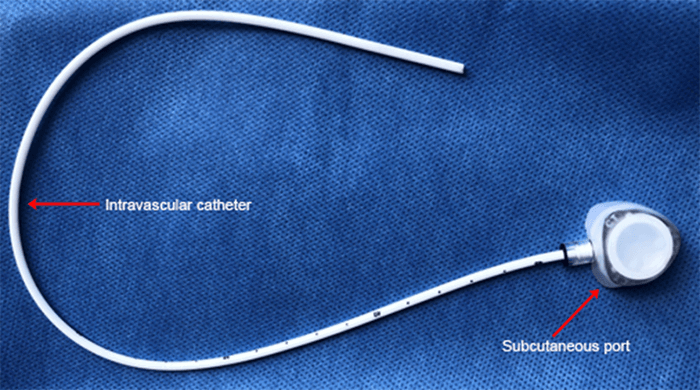
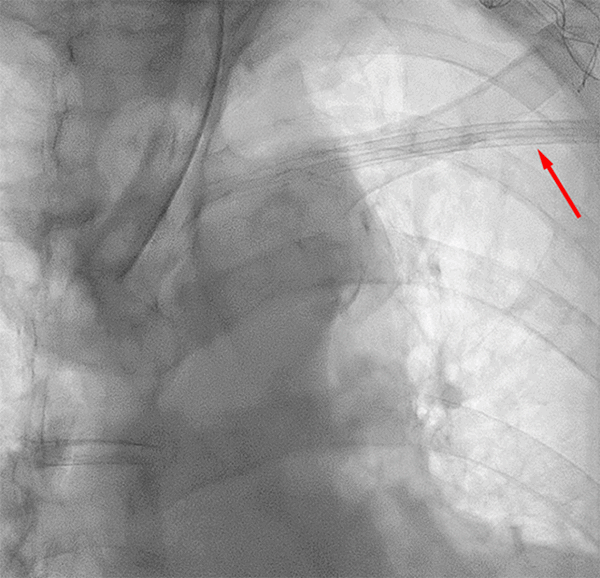
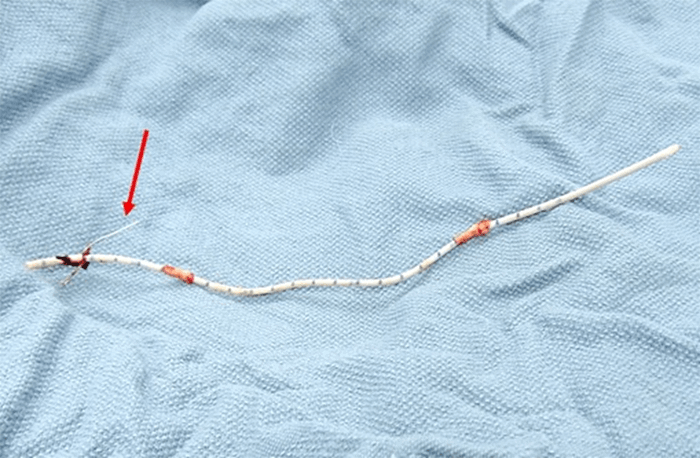
In the second case, a 71-year-old female with a history of a left internal jugular vein port placement nine years prior for the administration of chemotherapy for lung cancer was referred for surgical PAC removal because of complaints of discomfort at the reservoir site. Despite dissection down to the insertion site in the subclavian vein, the catheter was firmly adherent to the vasculature and unable to be removed despite robust manual traction. Subsequently, a consensus decision of the surgery and electrophysiology teams was made to revise the port-catheter system, an approach that we label a strategy of revision and abandonment. Thus, the reservoir was cut from the tubing and removed to relieve patient discomfort at the site, and a standard Medtronic pacemaker lead cap (Figure 3) was placed on the end of the tubing and secured with a 2-0 Silk tie.
The remaining portion of the catheter was replaced in the subcutaneous pocket, and the incision subsequently closed. After three months, the patient was doing well without any catheter-related complications.
Discussion
A retained intravenous catheter is a rare complication associated with the placement of totally implantable venous access devices. The incidence of retained catheters in pediatric populations has been reported between 0.3 and 2.2 percent. It has been associated with longer times since initial placement, type of medications administered (chemotherapeutic agents), and catheter materials (polyurethane versus silicone) 3–5). Although the adult literature is not as broad as in the pediatric population, the incidence of retained catheters is also low (<1 percent).6 A duration in situ exceeding two years is considered the most critical risk factor for retained catheters.7
In our cases, the ports remained in place for 11 and 9 years, respectively, representing the main factor predisposing to retention.
Presumably, fixation of long-term intravenous catheters is caused by calcification of the fibrin sheath formed around the catheter8,9 and bridging from the vein wall to the catheter.10
This rare complication represents a significant challenge as these catheters could be stuck within major vascular structures such as the jugular vein, subclavian vein, the superior vena cava, and even within the heart. When attempting to remove retained ports with simple manual traction, it is prudent to avoid forceful traction of the catheter to prevent complications such as fragmentation of the catheter with subsequent migration and vascular injuries.
Various alternative techniques have been used to facilitate the extraction of retained catheters when careful manual traction is not successful. They range in invasiveness from various endovascular methods (such as balloon dilators, loop-snares, basket retrievers, and pigtails11–13) to complicated open surgical interventions (such as intra-periosteal clavicle resections with subclavian venorrhaphy and neck exploration with jugular venorrhaphy.14) As these complex techniques are associated with increased potential for complications, it is essential to address the absolute need to extract the retained port in cases where it will not move with traction near the vein entry site.
In this paper, we describe two case reports involving two diametrically different strategies for managing retained PACs. The decision in both cases is guided by a multidisciplinary assessment of the need for PAC extraction. In the first case, in the presence of port infection, we decided to perform a complete port system extraction using cardiac electrophysiology extraction tools, a well-described approach.15 Evidence suggests that antibiotic treatment alone is not successful in eradicating the intra-vascular device infection. The formation of a biofilm on infected indwelling vascular catheters' surface has a critical role in bacterial antimicrobial resistance and recalcitrant infections as biofilm bacteria can survive high concentrations of antibiotics.16,17 Catheter-related infection is considered a strong indication for PAC removal and is the most common complication that results in device removal.18,19
In the second case, discomfort over the reservoir site was the only device-related symptom. In the absence of an absolute indication for complete port system extraction, a multidisciplinary decision was made to revise the reservoir and abandon the catheter, a technique that has not been described previously to the best of our knowledge. The port pocket was opened, the reservoir excised from the catheter tubing, and residual tubing was capped and secured in the pocket to prevent potential migration. This technique avoids potential risks of extraction, which could include vessel trauma and incomplete extraction from catheter fracture or dehiscence. Furthermore, removing the reservoir leads to normalization of the chest wall's topography. This reasonable cosmetic result may be important to some patients. The cosmetic results can be further enhanced by capping the catheter with a low-profile pacemaker lead cap.
Abandoning intravascular catheters has a theoretical risk for potential complications such as migration or thrombus in the future. In our opinion, catheter migration is unlikely as they are well fixed to the vein wall to the point of being unable to be manually removed. Nevertheless, to prevent the migration risk, we recommend suturing the catheter's external end on the pectoralis fascia. The long-term risk of thrombus formation is unknown but appears to be very low based on evidence from patients with retained pacemaker wires.20 In both cases, we reported, patients did well for approximately a decade without developing catheter-related thrombosis. Surveillance with Doppler ultrasound can be used to detect catheter-related clots in patients with elevated thrombotic risk.
Conclusion
A multidisciplinary evaluation is important to address the absolute need to extract retained ports in cases when they are difficult to remove with manual traction. Using cardiac pacemaker lead management as a model, strategies in dealing with retained ports can include complete removal of the reservoir-catheter system in the presence of infection versus abandoning non-functional or extraneous implants. A revision strategy consisting in the removal of the reservoir and capping of the catheter with a low-profile cardiac pacemaker lead is a safe option that can achieve satisfactory cosmetic results.
Lessons Learned
Timely removal of PACs that are no longer in use is important for preventing their retention. Cardiac pacemaker lead management could serve as a model in dealing with retained ports that cannot be extracted with manual traction, but only after a multidisciplinary assessment of the absolute need for their removal.
Authors
Hoffmeister PSa; Muñoz-Largacha JAb,c; Stolarski Ab,c; Kristo Gb,d
Author Affiliations
- Veterans Affairs Boston Healthcare System, Department of Cardiology, Boston, MA 02132
- Veterans Affairs Boston Healthcare System, Department of Surgery, Boston, MA 02132
- Boston University Medical Center, Department of Surgery, Boston, MA 02118
- Brigham and 'Women's Hospital; Harvard Medical School, Department of Surgery, Boston, MA 02115
Corresponding Author
Gentian Kristo, MD, MPH, FACS
Veterans Affairs Boston Healthcare System
Department of Surgery
1400 VFW Parkway
West Roxbury, Boston, MA 02132
E-mail: gentian.kristo@va.gov
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Wilson GJ, van Noesel MM, Hop WC, van de Ven C. The catheter is stuck: complications experienced during removal of a totally implantable venous access device. A single-center study in 200 children. J Pediatr Surg. 2006;41(10):1694-1698. doi:10.1016/j.jpedsurg.2006.05.065
- Bautista F, Gómez-Chacón J, Costa E, et al. Retained intravascular fragments after removal of indwelling central venous catheters: a single institution experience. J Pediatr Surg. 2010;45(7):1491-1495. doi:10.1016/j.jpedsurg.2010.02.001
- Aworanti OM, Linnane N, Tareen F, Mortell A. Incidence and outcome of retained Port-A-Cath fragments during removal. Pediatr Surg Int. 2017;33(7):777-781. doi:10.1007/s00383-017-4103-6
- Alzahrani K, Lejeune J, Lakhal W, et al. Polyurethane versus silicone port a cath: What's going on at removal?. J Pediatr Surg. 2018;53(7):1417-1419. doi:10.1016/j.jpedsurg.2017.06.025
- Chan BK, Rupasinghe SN, Hennessey I, Peart I, Baillie CT. Retained central venous lines (CVLs) after attempted removal: an 11-year series and literature review. J Pediatr Surg. 2013;48(9):1887-1891. doi:10.1016/j.jpedsurg.2013.01.050
- Vellanki VS, Watson D, Rajan DK, Bhola CB, Lok CE. The stuck catheter: a hazardous twist to the meaning of permanent catheters. J Vasc Access. 2015;16(4):289-293. doi:10.5301/jva.5000392
- Teague WJ, Fouad D, Munro FD, McCabe AJ. Complicated vascular access port removals: incidence, antecedents and avoidance. Pediatr Surg Int. 2015;31(9):859-864. doi:10.1007/s00383-015-3754-4
- Hoshal VL Jr, Ause RG, Hoskins PA. Fibrin sleeve formation on indwelling subclavian central venous catheters. Arch Surg. 1971;102(4):353-358. doi:10.1001/archsurg.1971.01350040115023
- Anderson MA, Poenaru D, Kamal I. Calcified catheter "cast": a rare complication of indwelling central venous catheters in infants. Pediatr Surg Int. 1998;13(8):610-612. doi:10.1007/s003830050418
- Forauer AR, Theoharis C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol. 2003;14(9 Pt 1):1163-1168. doi:10.1097/01.rvi.0000086531.86489.4c
- Vellanki VS, Watson D, Rajan DK, Bhola CB, Lok CE. The stuck catheter: a hazardous twist to the meaning of permanent catheters. J Vasc Access. 2015;16(4):289-293. doi:10.5301/jva.5000392
- Mousa AY, Gill G, Aburahma AF. New trick for removal of intravascular retained foreign body: a case report and review of literature. Vasc Endovascular Surg. 2014;48(1):55-57. doi:10.1177/1538574413510624
- Yang FS, Ohta I, Chiang HJ, Lin JC, Shih SL, Ma YC. Non-surgical retrieval of intravascular foreign body: experience of 12 cases. Eur J Radiol. 1994;18(1):1-5. doi:10.1016/0720-048x(94)90353-0
- Maizlin I, Carpentier H, Bliss D. Difficult extraction of long-term central venous catheters in children--case report. J Pediatr Surg. 2010;45(8):1720-1723. doi:10.1016/j.jpedsurg.2010.05.026
- De Lucia R, Segreti L, Soldati E, Di Cori A, Zucchelli G, Bongiorni MG. Port-a-Cath complicated by infection or migration not removed by manual traction: usefulness of cardiac pacing leads extraction techniques. Ann Vasc Surg. 2013;27(4):529-536. doi:10.1016/j.avsg.2012.07.015
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318-1322. doi:10.1126/science.284.5418.1318
- Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999-1007. doi:10.1128/AAC.45.4.999-1007.2001
- Narducci F, Jean-Laurent M, Boulanger L, et al. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol. 2011;37(10):913-918. doi:10.1016/j.ejso.2011.06.016
- Fischer L, Knebel P, Schröder S, et al. Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol. 2008;15(4):1124-1129. doi:10.1245/s10434-007-9783-z
- Van Den Akker-Berman LM, Pinzur S, Aydinalp A, et al. Uneventful 25-year course of an intracardiac intravenous catheter fragment in the right heart. J Interv Cardiol. 2002;15(5):421-423. doi:10.1111/j.1540-8183.2002.tb01080.x