Abstract
Background
A 69-year-old male patient presented with COVID-19 pneumonia and a rare case of concomitant pneumomediastinum and pneumoperitoneum before dying of multi-organ failure despite multimodal interventions.
Summary
The patient was a 69-year-old male presenting with malaise and worsening cough and dyspnea. He received multiple antimicrobials, COVID-19 adjuncts and respiratory adjuncts including extensive trial of CPAP/BiPAP before progressing to respiratory failure requiring intubation and intensive care. He was found radiographically to have pneumomediastinum with concomitant pneumomediastinum despite a benign abdominal exam and critically ill, but stable clinical picture. While pneumoperitoneum is typically caused by perforated viscus and treated with surgical intervention, this is a rare case of pneumoperitoneum caused by pneumomediastinum secondary to barotrauma from COVID-19 pneumonia that resolved spontaneously with prone positioning. This represents a new complication to add to the growing body of information surrounding this novel disease process.
Conclusion
Pneumomediastinum causing pneumoperitoneum is a rare clinical condition with a paucity of reports in literature. We present the first case of pneumomediastinum causing pneumoperitoneum as sequela of COVID-19 pneumonia that spontaneously resolved with prone positioning.
Key Words
COVID-19, pneumomediastinum, pneumoperitoneum, prone positioning
Case Description
Pneumomediastinum with simultaneous pneumoperitoneum is a rare event with few cases documented in literature. The first case was described by Lantsberg in 1992,1 with only six additional cases reported in literature to date.2 Herein we describe the first patient to our knowledge to develop this rare clinical entity as sequelae of COVID-19 pneumonia and with resolution after prone positioning while intubated.
The patient is a 69-year-old male with a history of coronary artery disease, hypertension, diabetes mellitus, and asthma who presented to the emergency room with fever and malaise. He had previously tested positive for COVID-19, demonstrated interval worsening on chest X ray, and was subsequently admitted for worsening dyspnea and cough. The patient developed subsegmental pulmonary emboli and was initially treated with a heparin drip which ultimately had to be stopped due to bleeding. The patient was treated with a variety of antimicrobials and other adjuncts for his COVID pneumonia including remdesivir, COVID-10 convalescent plasma, dexamethasone, and multiple antibiotics for presumed bacterial superinfection.
Despite this the patient’s respiratory status continued to deteriorate as he progressed from nasal cannula to high-flow nasal cannula to continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP). He remained on either CPAP or BiPAP for more than a week before ultimately requiring intubation on hospital day 15.
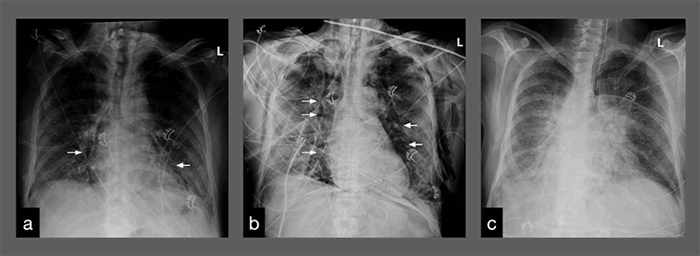
A chest X ray before intubation revealed evidence of pneumomediastinum. CT imaging confirmed pneumomediastinum and also demonstrated concomitant pneumoperitoneum without evidence of perforated viscus and bilateral apical pneumothoraces. At this time the patient was afebrile but had been fevering intermittently to 102.9°F. He was tachycardic to 133 bpm, on vasopressor support and on maximal settings of AC/VC+ ventilation. His abdomen was soft, non-distended and non-rigid; he had significant bilateral chest wall crepitus but no abdominal wall crepitus. He had a leukocytosis of 17.8 x106/µL and a stable anemia with hemoglobin/hematocrit of 9.7 g/dL/30.3 percent. His renal and hepatic panels were remarkable for an elevated blood glucose of 250 mg/dL and hypoalbuminemia of 1.9 g/dL. His coagulation panel showed PT of 20.3 s, PTT of 39.3 s and INR of 1.8. Inflammatory markers were a D-dimer of 1699 µg/mL, LDH of 702 u/L, ferritin of 1316 ng/mL, ESR of 115 mm/h and CRP of 12.65 mg/dL.
Ultimately, he was managed non-operatively with serial abdominal exams. His leukocytosis reached 20.3 x 106/µL the following morning before ultimately trending down and normalizing 24 hours later. He was placed in prone positioning for 16 hours nightly and received neuromuscular blockade with non-depolarizing agents starting that night, resulting in near complete resolution of biapical pneumothoraces and evidence of trace pneumomediastinum on the first day of proning. This necessitated that serial abdominal exams could be done only during the eight hours he was supine and not receiving paralytics; his abdominal exam did remain benign as above stated for the rest of his hospitalization. By the third and last day of prone positioning, a chest X ray demonstrated complete resolution of pneumothoraces and pneumomediastinum.
The patient continued to slowly but steadily decline over the course of hospital days 16 to 34. He began to more rapidly decline on hospital day 35, including desaturation to 40 percent, oliguria, and evidence of acute liver and renal failure with ALT > 3000 u/L and Cr > 2.0 mg/dL (baseline 0.7 mg/dL). The patient was DNR and ultimately died on hospital day 36.
Discussion
Multi-organ failure and specifically respiratory failure are well-documented sequelae of COVID-19 pneumonia.3 While the medical community and the world at large continue to combat the pandemic, there is a paucity of evidence-based therapeutic interventions. Various therapies have been used to this point including RNA-dependent RNA polymerase inhibitors, protease inhibitors, chloroquine, hydroxychloroquine, azithromycin, glycopeptides, antibody therapies, and convalescent plasma.4
This patient received antiretroviral and convalescent plasma therapy because of the reported in vivo success—albeit in the setting of small clinical case series and trials. The patient received remdesivir antiretroviral therapy, an RNA polymerase inhibitor that has shown potential for clinical improvement based on a compassionate-use study.5 He also received convalescent plasma, which has been proposed to suppress viremia using immunoglobulin antibodies present in the plasma of patients in the recovery phase of viral infection.6 There is precedent for this approach in other viral infections (SARS1 and MERS), and growing evidence in the setting of COVID-19.7,8
Despite multi-modal therapy, this patient’s respiratory failure progressively deteriorated requiring increasing respiratory support. This included high-flow nasal cannula, CPAP, and BiPAP, the latter two of which were alternated over the course of more than a week. He was ultimately transferred to the intensive care unit and intubated on hospital day 15. His initial chest films following intubation demonstrated new findings of bilateral pneumothoraces and pneumomediastinum, of which roughly 20 cases in the setting of COVID-19 pneumonia have been described in literature.9 However, this patient is the first documented case of pneumomediastinum with concomitant pneumoperitoneum in a COVID-positive patient.
Pneumomediastinum can be attributed to a multitude of causes. Some of the most common attributable causes include recent surgical manipulation or trauma, esophageal perforation, tracheobronchial injury or infection. The proposed pathogenesis of this patient’s mediastinum, however, is pneumatic dissection of paramediastinal tissues along the length of the esophagus through the diaphragmatic hiatus into the abdomen.
The Macklin effect is a well-described phenomenon of pneumomediastinum caused by alveolar rupture causing pneumatic dissection along bronchovascular sheaths and into the mediastinum.10 Barotrauma causes alveolar rupture, significant coughing, and use of positive-pressure respiratory adjuncts like CPAP/BiPAP may have been sources of barotrauma in this patient. The pneumomediastinum in this patient was discovered on a pre-intubation chest film, ruling out instrumentation of his airway as the initial cause.
Pneumoperitoneum most commonly occurs as a result of a perforated viscus, and typically requires surgical intervention for identification and repair of the perforation. In this case, our patient had radiographic evidence of both pneumomediastinum and pneumoperitoneum without any evidence of perforated viscus, and the pneumomediastinum appeared to dissect the paraesophageal tissues through the diaphragm and into the peritoneum (Figure 1). Because the most likely source of intra-peritoneal air was mediastinal and not a viscus perforation, coupled with the patient’s relatively benign abdominal exam, standard surgical interventions of diagnostic laparoscopy or midline laparotomy were not indicated.