Unfortunately, the patient developed rapidly progressing respiratory distress, was admitted to the intensive care unit (ICU) just a few hours after admission and required emergent intubation. Given this rapid change in the patient's condition and viability of the fetus at 35 weeks' gestation, the obstetrics and maternal-fetal medicine team felt that emergent cesarean section (C/S) delivery would improve pulmonary compliance and prevent fetal hypoxia. The patient underwent a noncomplicated C/S. She remained intubated postoperatively due to persistent respiratory acidosis and hypoxia. She received hydroxychloroquine, azithromycin, ribavirin, and received one dose of type-specific convalescent plasma. On postpartum day 2, she was transferred to our institution to manage severe hypoxemia from COVID-19 associated adult respiratory distress syndrome (ARDS). Initially, she was managed with lung-protective mechanical ventilation with a tidal volume of 6 ml/kg and PEEP of 10cmH2O, sedation with propofol and midazolam infusion, and paralysis with cisatracurium infusion but minimal improvement in tissue oxygenation. Subsequently, quadruple strength of continuous inhaled epoprostenol (Flolan) was added to her treatment plan, which improved tissue oxygenation for a short duration. Transthoracic echocardiogram revealed an ejection fraction of 60 to 64 percent with right ventricle (RV) enlargement and depression and pulmonary artery systolic pressure 56 mmHg consistent with ARDS and raised suspicions for pulmonary embolism given COVID-19 disease-associated hypercoagulability. After a discussion with the obstetrics team, the patient was started on heparin infusion for full anticoagulation with a partial thromboplastin time (PTT) goal of 60–80 per our institutional guidelines, along with prone positioning for severe hypoxemia.
On postpartum days 3 and 4, she developed refractory hypoxemia with the lung compliance worsened to 20–25 mL/H2O (normal 50–100 mL/H2O), PaO2 of 50 mmHg on FiO2 of 100 percent with PaO2/FiO2 ratio of <50 and PEEP of 14cmH2O despite treatment with the sixteen-hour session of prone positionings, inhaled epoprostenol, intravenous tocilizumab, and steroids. A pulmonary artery catheter was placed, which revealed normal pulmonary capillary wedge pressure. She also developed hemodynamic instability, which was treated with norepinephrine infusion. On postpartum day 5, the patient was evaluated for VV-ECMO for refractory hypoxemia associated with hemodynamic instability. On postpartum day 6, VV-ECMO was initiated by the cannulation of bilateral common femoral veins with a flow rate of 4.3 L/min, sweep 6 L/min, 3560 revolutions per minute (RPM), and FiO2 100 percent. Her oxygenation and hemodynamic status significantly improved, and PaO2 increased to 112 mmHg shortly after ECMO initiation. The arterial blood gas (ABG) target goal was to maintain the pH between 7.3 to 7.4 and oxygen saturation between 88 to 96 percent. On day 2 of ECMO, we slowly weaned the ECMO flow rate to 2.9 L/min, sweep to 0.5 L/min, 40 percent FiO2, and 2,500 RPM. She was extubated to high-flow nasal cannula on day 4 of ECMO and decannulated on day 9. A repeat transthoracic echocardiogram showed normal RV systolic function and resolution of pulmonary hypertension. Her hospital course progressed very well except for mild vaginal bleeding due to being anticoagulation with heparin infusion, which required suction dilation and curettage to evacuate blood clots. She recovered fully and was discharged home with two weeks of rivaroxaban per hematology recommendations.
Discussion
Pregnancy increases the risk of acquiring viral infection due to physiological changes during pregnancy.2 There is evidence of decreased T-cell numbers and immune cell activity, altering viral clearance.8,9 The evidence of severe novel coronavirus disease in pregnant and postpartum women is limited. Most reported cases of COVID-19 disease in pregnancy are described as asymptomatic infection or mild illness, which resolved without undergoing delivery.2–4 This case highlights that COVID-19 associated pneumonia can lead to ARDS, which can progress to refractory hypoxemia in pregnancy and the postpartum period. This case report adds to the growing body of evidence raising concerns for worse maternal outcomes in pregnant patients with COVID-19 disease.5,6
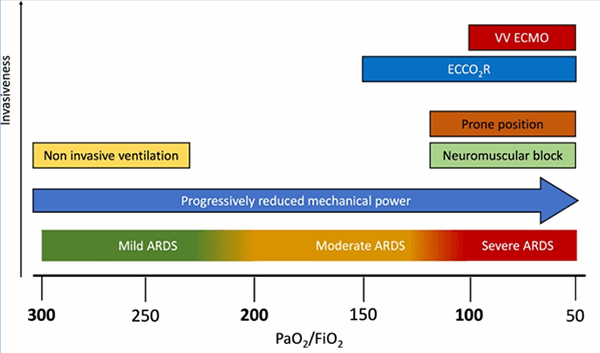
Pregnant patients with pneumonia are at increased risk of acute respiratory failure, need for mechanical ventilation, and admission to the intensive care unit. Complications from pneumonia include premature rupture of membrane, preterm labor, intrauterine fetal demise, intrauterine growth retardation, and neonatal death.8 Treatment of ARDS in pregnancy is generally similar to the general population, although some non-pregnant patients' strategies may not be acceptable to pregnant patients. Delivery of the fetus should be considered if maternal oxygenation does not improve with maximal therapy. In this case, maternal tissue oxygenation did not improve after the fetus's delivery despite following the algorithm of severe hypoxemic respiratory failure management (Figure 2).10 It was a challenging decision to initiate VV-ECMO on postpartum day six due to the risk of worsening postpartum vaginal bleeding. However, our patient's hypoxia was refractory to maximal medical therapy with inverse ratio ventilation, paralysis, steroids, prone positioning, and pulmonary vasodilators, leading to ECMO indication. After consultation with the obstetrics team and balancing the risk and benefits of therapeutic anticoagulation, ECMO was recommended as salvage therapy.