A) Low-power microscopy demonstrating cellular (Antoni A) and hypocellular regions (Antoni B); B) Medium-power microscopy demonstrating spindle cells with eosinophilic cytoplasm in schwannian stroma, C) High-power microscopy demonstrating schwannian cells mixed with stroma consisting of hyalinized vessels; D) Diffuse S100 positivity in schwannian cells
Discussion
Primary cardiac tumors are quite rare, with an incidence of approximately 0.1 percent. Ninety-five percent of cardiac neoplasms are instead metastases. Seventy-five percent of primary cardiac tumors are benign, and 50 percent of these are myxomas.1 Even of the remaining 50 percent, cardiac schwannomas, also known as neurilemomas, are exceedingly rare, few having been reported in the literature.2 These tumors are believed to arise from the cardiac neural plexus, branches of the autonomic nerves, and, as such, are predisposed to occur adjacent to the right atrium.3 Case reports have supported that these tumors can also develop neighboring the left atrium and in the atrioventricular groove.4,5 In evaluating these tumors, transthoracic echocardiography is often an appropriate first step; however, cardiac-protocol MRI is the ideal imaging modality for workup. It allows for evaluation of cardiac function and, as with other soft tissue neoplasms, provides the most information on tumor morphology, location, and relationship to surrounding structures.6 PET scans can help rule out metastatic disease and reinforce suspicion for malignancy. Still, as with many solid tumors, it does not have an established role in diagnosing cardiac schwannoma.7
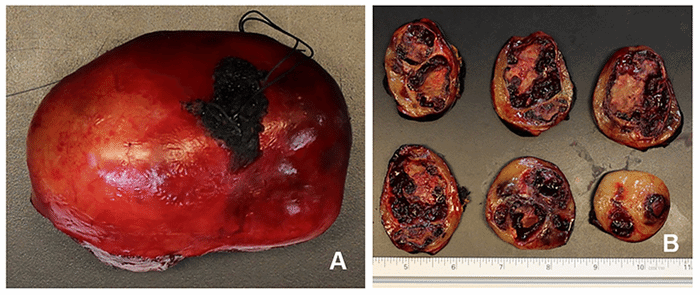
Despite the use of advanced imaging, diagnosis relies on tissue evaluation. Furthermore, though frozen sampling can aid in decision-making, said diagnosis is dependent on a more thorough histologic evaluation, ideally with IHC.8 This is supported by our reported case where both MRI and frozen pathology were concerning for sarcoma, but final pathology diagnosed schwannoma. This discrepancy occurs because schwannomas exhibit both gross and microscopic heterogeneity, leading to a similar appearance to sarcoma on imaging. Image-guided core or incisional biopsies are also subject to sampling bias. Schwannomas are composed of biphasic architecture consisting of Antoni A and B patterns, where elongated fascicles represent the former with dense palisading cellularity (Verocay bodies) and the latter by loose cellular meshworks and microcysts. Histologically, the tumors are also characterized by fibrous capsules surrounding nerve-derived cells.9 This neural-crest-derived tissue allows schwannomas to be identified with IHC by S-100 binding.1
Cardiac schwannomas are too rare to have measured outcomes, but, in general, the prognosis associated with benign cardiac tumors is excellent. Given that even these benign tumors can cause local anatomic and physiologic disruption, prognosis and cure are correlated with the adequacy of resection.10 A pathologic complete (R0) resection can be difficult to achieve given high-value anatomic and physiologic real estate. Case reports have described the need for cardiopulmonary bypass, coronary bypass grafting, and pericardial patching to complete resection.2,4,11 One must balance the risk of these aggressive steps with the risk of incomplete resection. Despite microscopically positive margins in our specimen (R1 resection), resection was grossly total and believed to be the maximal allowable given the high risk of atrioventricular groove disruption with further attempts to gain margin.
Conclusion
The presentation describing an AV groove cardiac schwannoma emphasizes the problematic diagnosis and complex decision-making surrounding mediastinal tumors found incidentally, mainly when surgical resection is high-risk. Diagnostic studies may aid in creating a differential and evaluating associated anatomy, but resection and resultant microscopic examination is required for a proper diagnosis. In preparing for surgical intervention, one must be ready to provide cardiopulmonary support and alter the intraoperative course based upon changes in presumptive diagnosis and the associated balance of risk and benefit.
Lessons Learned
The decision-making surrounding any incidentally found tumor is complex and particularly difficult when initial evaluation cannot rule out malignant disease. With cardiac schwannomas, surgical resection is necessary to confirm a diagnosis but is often challenging and requires a careful balancing of the surgical risks and benefits.
Authors
Platz JJa; Patel VAb; Hermsen JLc; DeCamp MMc
Author Affiliations
- Division of Thoracic Surgery, Saint Louis University School of Medicine, Saint Louis, MO 63110
- Department of Pathology, University of Wisconsin School of Medicine and Public Health, Madison, WI 53792
- Division of Cardiothoracic Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI 53792
Corresponding Author
Malcolm M. DeCamp, MD, FACS
University of Wisconsin Hospital and Clinics, H4/340 CSC
600 Highland Avenue
Madison, WI 53792
Phone: (608) 263-6551
E-mail: decamp@surgery.wisc.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no financial relationships or in-kind support to disclose.
Received: April 23, 2019
Revision Received: September 17, 2020
Accepted for Publication: November 17, 2020
References
- Stefansson K, Wollmann R, Jerkovic M. S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. Am J Pathol. 1982;106(2):261-268.
- Hashimoto T, Eguchi S, Nakayama T, Ohzeki H, Hayashi J. Successful removal of massive cardiac neurilemoma with cardiopulmonary bypass. Ann Thorac Surg. 1998;66(2):553-555. doi:10.1016/s0003-4975(98)00473-1
- D'Amato N, Correale M, Ireva R, Di Biase M. A rare cause of acute heart failure: malignant schwannoma of the pericardium. Congest Heart Fail. 2010;16(2):82-84. doi:10.1111/j.1751-7133.2009.00124.x
- Hwang SK, Jung SH. Schwannoma of the heart. Korean J Thorac Cardiovasc Surg. 2014;47(2):141-144. doi:10.5090/kjtcs.2014.47.2.141
- Sirlak M, Uymaz OK, Taşoz R, Erden E, Ozyurda U, Akalin H. Primary benign schwannoma of the heart. Cardiovasc Pathol. 2003;12(5):290-292. doi:10.1016/s1054-8807(03)00076-0
- Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27(5):459-469. doi:10.1007/s00270-004-0123-4
- Saponara M, Ambrosini V, Nannini M, et al. 18F-FDG-PET/CT imaging in cardiac tumors: illustrative clinical cases and review of the literature. Ther Adv Med Oncol. 2018;10:1758835918793569. Published 2018 Aug 30. doi:10.1177/1758835918793569
- Early SA, McGuinness J, Galvin J, Kennedy M, Hurley J. Asymptomatic schwannoma of the heart. J Cardiothorac Surg. 2007;2:1. Published 2007 Jan 4. doi:10.1186/1749-8090-2-1
- Kumar V, Abbas A, Aster J. Robbins & Cotran Pathologic Basis of Disease, 9th ed. Elsevier Saunders 2014.
- Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219-228. doi:10.1016/S1470-2045(05)70093-0
- Koujanian S, Pawlowicz, Landry D. Benign cardiac schwannoma: a case report. Human Pathology: Case Reports 2017;8:24-6.