Small intestinal mucosa with partially denuded epithelium, reactive epithelial changes, and admixed acute and chronic inflammatory infiltrates, A) 40x magnification and B) 100x magnification; ulcerated small intestinal mucosa with neutrophilic and lymphoplasmacytic inflammation in background of stromal edema and prominent congested blood vessels, C) 40x magnification and D) 100x magnification
Detailed pathologic findings included the following details: on gross examination, serosal surfaces of the resected jejunum were variegated, diffusely hemorrhagic, and focally shaggy. The mucosal surfaces were congested and attenuated with a few normal intestinal folds. There were no masses or lesions. On histological examination, the small intestinal mucosa was ulcerated with denuded epithelium and extensive mixed inflammatory infiltrates, with areas of erosion also present. There were prominent congested blood vessels in the submucosa with stromal edema. The serosal surface of the small intestine had dense fibroconnective tissue, consistent with serosal adhesions. 90Y microspheres or neoplasm were not identified.
Discussion
Yttrium-90 (90Y) transarterial radioembolization (TARE), a form of intra-arterial brachytherapy, is a notable option in the treatment armamentarium for liver malignancies, including hepatocellular carcinoma (HCC) and metastatic neuroendocrine tumors (NET). First used in the 1960s to manage primary HCC and metastatic NET not amenable to surgical resection, 90Y TARE has demonstrated favorable tumor response and patient survival that stands the test of time.1‒6 Overall, 90Y TARE is considered a safe therapy. In a recent meta-analysis comprising 870 patients with NET liver metastases treated with 90Y TARE, complications were reported in less than 1 percent of patients.7 Complications may result from collateral radiation damage or nontarget deposition of the 90Y radioactive microspheres, and include radiation pneumonitis, gastritis, cholecystitis, duodenal ulceration, post-procedure hepatic insufficiency, and liver failure.8‒10 Although rare, non-neoplastic gastric and duodenal ulcers have been described following 90Y TARE of hepatic tumors; however, there are no reports of jejunal or pancreaticobiliary reconstruction limb ulcers to our knowledge.10,11 Here, we report a case of a perforated jejunal ulcer in a patient with post-pancreaticoduodenectomy surgical anatomy and metastatic gastrinoma recurrence treated by 90Y TARE.
We describe a case of pancreaticobiliary reconstruction limb ulcers that occurred after 90Y TARE to an adjacent liver segment in a patient with post-pancreaticoduodenectomy surgical anatomy. In our literature review, this is the first report of ulceration in the jejunum of the pancreaticobiliary reconstruction limb following 90Y TARE.
90Y TARE is a widely utilized therapeutic modality for treating liver malignancies not amenable to resection or ablation, including NET metastases as in our patient.9,11,12 With over 50 years of data available, 90Y TARE has demonstrated effective locoregional tumor control and beneficial long-term oncologic outcomes for both primary and metastatic hepatic malignancies.1,6,7 For NET metastases specifically, 90Y TARE has been associated with symptom relief, favorable disease control, and improved survival, with 78 percent of patients exhibiting tumor response or stable disease and a 72.5 percent one-year survival rate.7,14
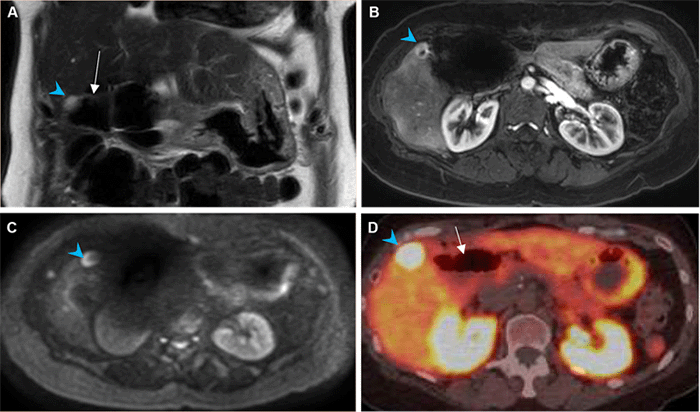
The toxicity of radioembolization derives from what also provides its therapeutic effect: radiation.15 The interventional radiologist must select the appropriate hepatic arterial inflow for radioembolization to minimize this toxicity to adjacent normal tissue. Improvements in technology, technique, and experience have enabled more selective administration, thus minimizing adverse events.11,16 However, there are still complications from radiation-induced collateral liver damage, with up to 77 percent of patients exhibiting grade II or III liver toxicity one year after treatment, based on National Cancer Institute Common Toxicity Criteria for Acute Events.8 While gastric and duodenal ulcers are the most commonly reported extrahepatic complications of 90Y TARE, they are rare, with recent literature indicating a prevalence of less than 5 percent.17‒19 These are thought to be caused by nontarget microsphere accumulation in the gastrointestinal submucosa resulting in tissue and small vessel damage.10,11,20 In this patient's case, a subsegmental arterial branch supplying the segment V tumor was specifically identified and targeted to deliver the 90Y microspheres, with no evidence of nontarget delivery.
The development of the jejunal ulceration in our patient was a result of a "perfect storm": the segment V hepatic recurrence was located in a superficial, subcapsular location; a dedicated branch of the hepatic artery was identified to be supplying the tumor on the mapping angiogram made this lesion amenable to super-selective, high-dose delivery of the 90Y microspheres; and the patient's altered post-pancreaticoduodenectomy anatomy placed the affected jejunal segment directly adjacent to the radiated liver. The jejunal ulceration thus resulted not from nontarget microsphere deposition but an ablative radiation dose close to the small bowel, described at our institution as "shine-through radiation." The average tissue penetration of the beta energy from 90Y TARE is 2 mm to 3 mm, up to a maximum of 11 mm. To this end, a retrospective study of 97 patients undergoing non-ablative, conventional radioembolization to the left hepatic lobe demonstrated the safety of the therapy when delivered near the gastric wall.21
In our patient's case, the ulcerated jejunum was not just close to the liver metastasis but was found intraoperatively to be adherent to it. Of note, there is limited experience regarding special considerations for 90Y TARE in patients after pancreaticoduodenectomy. In a cohort of 33 patients receiving 90Y treatment for liver metastases from pancreatic adenocarcinoma, there were no reports of gastrointestinal ulceration in ten patients who had undergone prior pancreatic resection. However, whether these patients had undergone pancreaticoduodenectomy or the location of the liver metastases was not specified.22
Furthermore, a higher radiation dose than conventional TARE was used in this case to perform an ablative radioembolization, or radiation segmentectomy, in which increased doses administered to two or fewer liver segments are considered curative.23 In short, the 'patient's post-pancreaticoduodenectomy anatomy placing the jejunum directly adjacent to the superficial liver tumor, combined with the high radiation dose afforded by ablative TARE, is the likely cause of this unique complication.
Our patient developed two sets of jejunal ulcers in succession from distinct etiologies. She initially formed jejunal ulcers adjacent to the DJ anastomosis due to her gastrinoma recurrence and its associated acid hypersecretion, which improved with PPI and ultimately resolved with 90Y TARE treatment of the recurrence. She subsequently developed new ulcers adjacent to the HJ anastomosis following 90Y TARE therapy, when serum chromogranin A and gastrin levels were low. It is unlikely that the segment of jejunum affected had been previously damaged by stomach acid hypersecretion as it was in the afferent limb, considerably distant relative to the DJ anastomosis. Of note, there was a low threshold for performing endoscopy in this patient given concern for recurrent cancer, which may have led to earlier, serendipitous detection of the post-90Y ulcers.
Conclusion
We present the first known case of a perforated pancreaticobiliary reconstruction limb ulcer following 90Y TARE and requiring segmental jejunal resection in a post-pancreaticoduodenectomy patient. When considering 90Y therapy follow-up, patients like ours with aberrant anatomy such that a segment of the small bowel is directly adjacent to the 90Y treatment bed warrant special attention.
Lessons Learned
While gastrointestinal ulceration is a rare complication of ablative, yttrium-90 transarterial radioembolization for treating liver malignancies, patients with altered intestinal anatomy present a unique challenge. Pancreaticobiliary reconstruction limb ulceration is a potential complication of 90Y radiation segmentectomy in patients with prior pancreaticoduodenectomy.
LKD, KPL, and JOP contributed to the conception of the work. All authors contributed to the interpretation of the patient information, including laboratory values, endoscopic images, radiologic images, operative findings, and histopathology findings. LKD, KPL, CW, WM, and JOP drafted the article and/or substantively revised it. All authors read and approved the final version to be submitted.
References
- Saini A, Wallace A, Alzubaidi S, et al. History and evolution of yttrium-90 radioembolization for Hepatocellular Carcinoma. J Clin Med. 2019;8(1):55. Published 2019 Jan 7. doi:10.3390/jcm8010055
- Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg. 1965;162(2):267-278. doi:10.1097/00000658-196508000-00018
- Simon N, Warner RR, Baron MG, Rudavsky AZ. Intra-arterial irradiation of carcinoid tumors of the liver. Am J Roentgenol Radium Ther Nucl Med. 1968;102(3):552-561. doi:10.2214/ajr.102.3.552
- Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83(3):887-894. doi:10.1016/j.ijrobp.2011.07.041
- National Comprehensive Cancer Network. Neuroendocrine and adrenal tumors clinical practice guidelines (Version 1.2019). https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J. 2010;16(2):163-175. doi:10.1097/PPO.0b013e3181d7e8cf
- Jia Z, Wang W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur J Radiol. 2018;100:23-29. doi:10.1016/j.ejrad.2018.01.012
- Tomozawa Y, Jahangiri Y, Pathak P, et al. Long-term toxicity after transarterial radioembolization with yttrium-90 using resin microspheres for neuroendocrine tumor liver metastases. J Vasc Interv Radiol. 2018;29(6):858-865. doi:10.1016/j.jvir.2018.02.002
- Sacco R, Mismas V, Marceglia S, et al. Transarterial radioembolization for hepatocellular carcinoma: An update and perspectives. World J Gastroenterol. 2015;21(21):6518-6525. doi:10.3748/wjg.v21.i21.6518
- Blesl A, Brcic I, Jaschke W, Öfner D, Fickert P, Plank J. Chronic gastric ulcer disease complicating selective internal radiation therapy (SIRT) in a patient with cholangiocellular carcinoma. SIRT induzierte chronische gastroduodenale Vaskulopathie. Z Gastroenterol. 2019;57(11):1304-1308. doi:10.1055/a-1016-3698
- Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17(10):1571-1593. doi:10.1097/01.RVI.0000236744.34720.73
- Egger ME, Armstrong E, Martin RC 2nd, et al. Transarterial chemoembolization vs. radioembolization for neuroendocrine liver metastases: a multi-institutional analysis. J Am Coll Surg. 2020;230(4):363-370. doi:10.1016/j.jamcollsurg.2019.12.026
- Saini A, Wallace A, Alzubaidi S. History and evolution of Yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med. 2019;8:E55. doi: 10.3390/jcm8010055
- Jia Z, Paz-Fumagalli R, Frey G, Sella DM, McKinney JM, Wang W. Single-institution experience of radioembolization with yttrium-90 microspheres for unresectable metastatic neuroendocrine liver tumors. J Gastroenterol Hepatol. 2017;32(9):1617-1623. doi:10.1111/jgh.13752
- Lee EJ, Chung HW, Jo JH, So Y. Radioembolization for the treatment of primary and metastatic liver cancers. Nucl Med Mol Imaging. 2019;53(6):367-373. doi:10.1007/s13139-019-00615-9
- Padia SA, Johnson GE, Horton KJ, et al. Segmental yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol. 2017;28(6):777-785.e1. doi:10.1016/j.jvir.2017.02.018
- Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198. Published 2014 Jul 29. doi:10.3389/fonc.2014.00198
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624-1636. doi:10.1016/S1470-2045(17)30683-6
- Padia SA, Lewandowski RJ, Johnson GE, et al. Radioembolization of hepatic malignancies: background, quality improvement guidelines, and future directions. J Vasc Interv Radiol. 2017;28(1):1-15. doi:10.1016/j.jvir.2016.09.024
- Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25 Suppl 1:S41-S55. doi:10.1148/rg.25si055515
- Gates VL, Hickey R, Marshall K, et al. Gastric injury from (90)Y to left hepatic lobe tumors adjacent to the stomach: fact or fiction?. Eur J Nucl Med Mol Imaging. 2015;42(13):2038-2044. doi:10.1007/s00259-015-3122-6
- Kim AY, Frantz S, Brower J, Akhter N. Radioembolization with yttrium-90 microspheres for the treatment of liver metastases of pancreatic adenocarcinoma: a multicenter analysis. J Vasc Interv Radiol. 2019;30(3):298-304.e2. doi:10.1016/j.jvir.2018.09.020
- Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers (Basel). 2019;11(8):1085. Published 2019 Jul 31. doi:10.3390/cancers11081085
Authors
Dickerson LKa; Labadie KPa; Brentnall TAb; Saunders MDb; Wu Bc; Yeh MMc; Johnson GEd; Monsky WLd; Park JOa
Author Affiliations
- Department of Surgery, Hepatopancreaticobiliary Surgical Oncology Division, University of Washington Medical Center, Seattle, WA 98195
- Department of Medicine, Gastroenterology Division, University of Washington Medical Center, Seattle, WA 98195
- Department of Laboratory Medicine and Pathology, University of Washington Medical Center, Seattle, WA 98195
- Department of Radiology, Interventional Radiology Division, University of Washington Medical Center, Seattle, WA 98195
Corresponding Author
James O. Park, MD, FACS
Department of Surgery
University of Washington Medical Center
1959 NE Pacific Street
Box 356410
Seattle, WA, 98195
Phone: 206-685-4672
Email: jopark@uw.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 20, 2021
Revision received: October 1, 2021
Accepted: November 1, 2021