Discussion
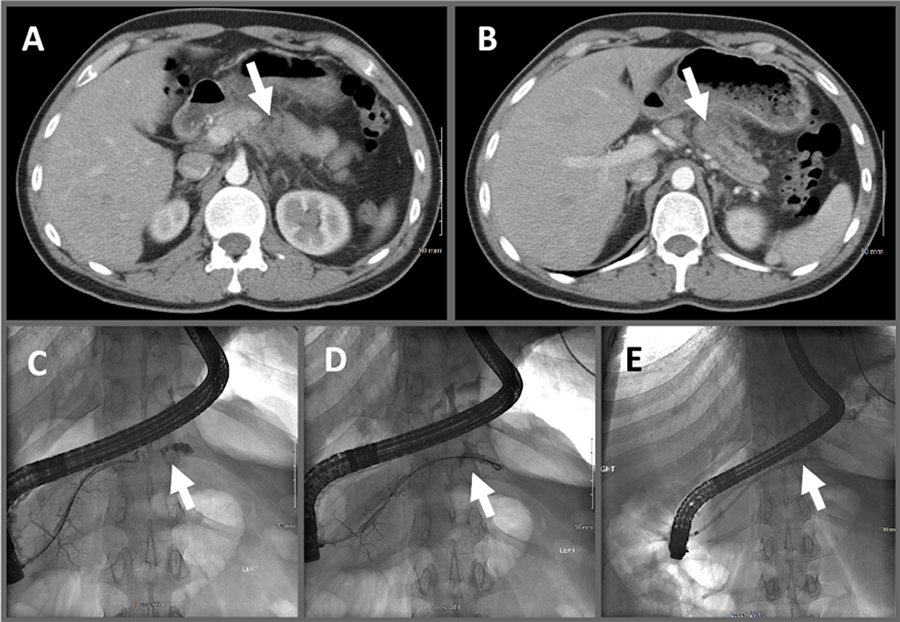
We present the intersection of multiple rare phenomena, with a sporadic MMR-d PDAC found in a 30-year-old who presented with trauma-induced pancreatitis. Risk factors for PDAC include advanced age, smoking, chronic alcoholism, chronic pancreatitis, obesity, diabetes, family history, and H pylori infection.7‒16 Although he was a heavy smoker, he did not exhibit any other risk factors. It is well documented that chronic pancreatitis can increase the risk of pancreatic cancers,9,10,13,15,17 and it may be difficult to discern adenocarcinomas from chronic pancreatitis radiographically.18 Trauma can predispose patients to chronic pancreatitis.19‒21 However, the incidence of trauma-induced pancreatitis leading to PDAC is unknown. Before our patient's mild abdominal trauma from wrestling, he had no symptoms or complaints. Disconnected-duct syndrome would more typically be seen after severe trauma, not mild trauma. We suspect that the tumor may have predisposed him to a fracture of his pancreas at that site, which was the inciting factor for pancreatitis, not the converse.
PDAC in the young (≤65 years old) is rare.3,22‒24 In a single-institution cohort study of 3202 biopsy-proven PDAC cases from Memorial Sloan Kettering Cancer Center, 136 (4.4%) were ≤45 years old, with only 4 (0.1%) of these between 20-29 and 38 (1.1%) between 30-39 years old.24 Like our patient, the majority (90%) of this young cohort did not have a family history of pancreatic cancer. Similar to the elderly population, the minority (25.7%) of young PDAC patients presented with early-stage disease and underwent resection. However, compared to two recent national clinical trials showing a median of 18 to 22 months overall survival (OS) after resection of early-stage PDAC, these patients fared better after resection (median OS 41.8 months), possibly due to fewer comorbidities.24 Unfortunately, early diagnosis is achieved the minority of the time since most are asymptomatic or display nonspecific, vague symptoms until late stages.14,17 Incidental findings requiring urgent follow-up are seen in up to one-third of patients undergoing CT scans for trauma, although pancreatic masses and cysts are seen only 0.3% and 0.2% of the time, respectively.25 There are multiple reports identifying pancreatic cysts,25‒27 pseudocysts,28 and in particular solid pseudopapillary neoplasm (Franz's tumor)29‒32 following trauma; however, there is scant literature linking PDAC and trauma.
Interestingly, our patient's tumor showed MMR-d with loss of nuclear MSH2 and MSH6 expression by IHC as well as microsatellite instability (MSI-H), which are typical of Lynch syndrome (LS).33,34 By age 70, 3.7% of LS patients will develop PDAC, compared to 1.5% for the general population.34 In our patient, however, germline analysis did not identify any hereditary cancer syndrome, and further molecular analysis of the tumor did not find double somatic mutations to explain the loss of MSH2 and MSH6 in the tumor. We did find a somatic MLH1 mutation, although this is not associated with loss of MSH2 and MSH6 by IHC. The literature varies on the exact proportion of MMR-d/MSI-H PDAC, but in several recent large series with modern detection techniques, 1 to 2% of all PDAC were found to have this phenotype.35 This is more often seen in medullary and acinar cell carcinomas of the pancreas,35 while our patient showed signet-ring cell and mucinous features with areas of ductal differentiation. There is debate35 on the proportion of MMR-d PDAC arising from germline mutations (i.e., LS), ranging from all (7 of 7 MMR-d)36 to none (0 of 4)37 and varies depending on the patient population tested and detection methods. Overall, while multiple mechanisms to achieve an MMR-d/MSI-H PDAC exist, the common phenotype result is a high mutational burden, postulated to lead to increased neoantigen presentation to infiltrating cytotoxic T cells.35 Checkpoint inhibition (immune therapy) is increasingly being utilized in MSI-H cancers, including PDAC,38‒45 with proof of principle seen in a recent study in which the cohort of eight PDACs receiving pembrolizumab had two complete responses, three partial responses, and one with stable disease.
Conclusion
PDAC is rare in the young. Incidental radiographic findings at trauma or during workup for other conditions are common and should be critically evaluated for malignant potential. Pancreatitis may be caused by trauma, and chronic pancreatitis is a risk factor for PDAC. Here we report a case in which disconnected-duct syndrome and chronic pancreatitis following mild abdominal trauma uncovered the diagnosis of PDAC in a 30-year-old male. Since the patient's tumor demonstrated an MMR-d/MSI-H phenotype, he was able to receive immunotherapy at the time of his recurrence.
Lessons Learned
It is important to closely follow patients with incidentally discovered pancreatic abnormalities, particularly those that develop pancreatitis, as this can be an early sign of malignancy, regardless of age.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet Gastroenterol Hepatol. 2020 Mar;5(3):e2]. Lancet Gastroenterol Hepatol. 2019;4(12):934-947. doi:10.1016/S2468-1253(19)30347-4
- American Cancer Society: Cancer Facts & Statistics. American Cancer Society | Cancer Facts & Statistics. https://cancerstatisticscenter.cancer.org/#!/data-analysis/Prob_DevCan.
- Goodman M, Willmann JK, Jeffrey RB. Incidentally discovered solid pancreatic masses: imaging and clinical observations. Abdom Imaging. 2012;37(1):91-97. doi:10.1007/s00261-011-9720-2
- Fischer TD, Gutman DS, Hughes SJ, Trevino JG, Behrns KE. Disconnected pancreatic duct syndrome: disease classification and management strategies. J Am Coll Surg. 2014;219(4):704-712. doi:10.1016/j.jamcollsurg.2014.03.055
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549-554. doi:10.1016/0092-8674(88)90571-5
- Korpela T, Udd M, Mustonen H, et al. Association between chronic pancreatitis and pancreatic cancer: A 10-year retrospective study of endoscopically treated and surgical patients. Int J Cancer. 2020;147(5):1450-1460. doi:10.1002/ijc.32971
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20(2):197-209. doi:10.1016/j.bpg.2005.10.001
- Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328(20):1433-1437. doi:10.1056/NEJM199305203282001
- Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51(6):849-852. doi:10.1136/gut.51.6.849
- McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846-4861. doi:10.3748/wjg.v24.i43.4846
- Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381(1):269-277. doi:10.1016/j.canlet.2016.07.022
- Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24(3):349-358. doi:10.1016/j.bpg.2010.02.007
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. doi:10.14740/wjon1166
- Shimosegawa T, Kume K, Satoh K. Chronic pancreatitis and pancreatic cancer: prediction and mechanism. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S23-S28. doi:10.1016/j.cgh.2009.07.042
- Barone E, Corrado A, Gemignani F, Landi S. Environmental risk factors for pancreatic cancer: an update. Arch Toxicol. 2016;90(11):2617-2642. doi:10.1007/s00204-016-1821-9
- Agarwal S, Sharma S, Gunjan D, et al. Natural course of chronic pancreatitis and predictors of its progression. Pancreatology. 2020;20(3):347-355. doi:10.1016/j.pan.2020.02.004
- Ruszniewski P, Malka D, Hammel P, et al. The diagnostic dilemmas in discrimination between pancreatic carcinoma and chronic pancreatitis. Gut. 2004;53(5):771.
- Bradley EL 3rd. Chronic obstructive pancreatitis as a delayed complication of pancreatic trauma. HPB Surg. 1991;5(1):49-60. doi:10.1155/1991/73834
- Sharbidre KG, Galgano SJ, Morgan DE. Traumatic pancreatitis. Abdom Radiol (NY). 2020;45(5):1265-1276. doi:10.1007/s00261-019-02241-7
- Gholson CF, Sittig K, Favrot D, McDonald JC. Chronic abdominal pain as the initial manifestation of pancreatic injury due to remote blunt trauma of the abdomen. South Med J. 1994;87(9):902-904. doi:10.1097/00007611-199409000-00008
- Bracci PM, Wang F, Hassan MM, Gupta S, Li D, Holly EA. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control. 2009;20(9):1723-1731. doi:10.1007/s10552-009-9424-x
- Howlader N NA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review. In. National Cancer Institute. Bethesda, MD.
- Duffy A, Capanu M, Allen P, et al. Pancreatic adenocarcinoma in a young patient population--12-year experience at Memorial Sloan Kettering Cancer Center. J Surg Oncol. 2009;100(1):8-12. doi:10.1002/jso.21292
- Barrett TW, Schierling M, Zhou C, et al. Prevalence of incidental findings in trauma patients detected by computed tomography imaging. Am J Emerg Med. 2009;27(4):428-435. doi:10.1016/j.ajem.2008.03.025
- Debi U, Kaur R, Prasad KK, Sinha SK, Sinha A, Singh K. Pancreatic trauma: a concise review. World J Gastroenterol. 2013;19(47):9003-9011. doi:10.3748/wjg.v19.i47.9003
- Leppäniemi A, Haapiainen R, Kiviluoto T, Lempinen M. Pancreatic trauma: acute and late manifestations. Br J Surg. 1988;75(2):165-167. doi:10.1002/bjs.1800750227
- Connor K, Yawathe M, Harrison E. Pancreatic Cyst After Trauma in a Young Female. Gastroenterology. 2016;150(3):e3-e4. doi:10.1053/j.gastro.2015.07.047
- Cervantes-Monteil F, Florez-Zorrilla C, Alvarez-Martínez I. Tumor pseudopapilar sólido-quístico de páncreas. Presentación aguda postraumática. Reporte de un caso y revisión de la literatura [Solid-cystic pseudopapillary tumor of the pancreas: acute post-traumatic presentation. Case report and review of the literature]. Rev Gastroenterol Mex. 2002;67(2):93-96.
- Hansson B, Hubens G, Hagendorens M, Deprettere A, Colpaert C, Eyskens E. Frantz's tumour of the pancreas presenting as a post-traumatic pancreatic pseudocyst. Acta Chir Belg. 1999;99(2):82-84.
- Vergauwen W, Op de Beeck B, Hagendorens M, Wojciechowski M, Vaneerdeweg W, Ramet J. A solid pseudopapillary tumour of the pancreas presenting after an abdominal trauma. Acta Chir Belg. 2010;110(3):390-393. doi:10.1080/00015458.2010.11680642
- Lévy P, Bougaran J, Gayet B. Carcinose péritonéale diffuse d'une tumeur pseudo-papillaire et solide du pancréas. Rôle d'un traumatisme abdominal [Diffuse peritoneal carcinosis of pseudo-papillary and solid tumor of the pancreas. Role of abdominal injury]. Gastroenterol Clin Biol. 1997;21(10):789-793.
- Bujanda L, Herreros-Villanueva M. Pancreatic cancer in Lynch syndrome patients. J Cancer. 2017;8(18):3667-3674. Published 2017 Oct 11. doi:10.7150/jca.20750
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302(16):1790-1795. doi:10.1001/jama.2009.1529
- Lupinacci RM, Bachet JB, André T, Duval A, Svrcek M. Pancreatic ductal adenocarcinoma harboring microsatellite instability / DNA mismatch repair deficiency. Towards personalized medicine. Surg Oncol. 2019;28:121-127. doi:10.1016/j.suronc.2018.11.019
- Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326-1336. doi:10.1158/1078-0432.CCR-17-3099
- Humphris JL, Patch AM, Nones K, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152(1):68-74.e2. doi:10.1053/j.gastro.2016.09.060
- Hong SM, Park JY, Hruban RH, Goggins M. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med. 2011;135(6):716-727. doi:10.5858/2010-0566-RA.1
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38(1):1-10. doi:10.1200/JCO.19.02105
- Liang D, Shi S, Liang C, et al. Mismatch repair status as a beneficial predictor of fluorouracil-based adjuvant chemotherapy for pancreatic cancer. Surgery. 2018;163(5):1080-1089. doi:10.1016/j.surg.2017.12.009
- Riazy M, Kalloger SE, Sheffield BS, et al. Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod Pathol. 2015;28(10):1383-1389. doi:10.1038/modpathol.2015.89
- Sahin IH, Lowery MA, Stadler ZK, et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hepatol. 2016;10(8):893-905. doi:10.1586/17474124.2016.1153424
- Dell'Aquila E, Fulgenzi CAM, Minelli A, et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget. 2020;11(10):924-941. Published 2020 Mar 10. doi:10.18632/oncotarget.27518
- Eatrides JM, Coppola D, Al Diffalha S, Kim RD, Springett GM, Mahipal A. Microsatellite instability in pancreatic cancer. J Clin Oncol (2016) 34 (15_suppl):e15753. doi: 10.1200/JCO.2016.34.15_suppl.e15753
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi:10.1126/science.aan6733
Authors
Harper Ma; Du Jb; Schaberg Kb; Patel Rc; Moss Jc; Kolesar Jde; Nandakumar Kb; Pickarski Je; Pandalai Pa; McGrath Pa; Kim Ja; Cavnar Ma
Author Affiliations
- Department of Surgery, University of Kentucky College of Medicine, Lexington, KY 40508
- Department of Pathology, University of Kentucky College of Medicine, Lexington, KY 40508
- Department of Medicine, University of Kentucky College of Medicine, Lexington, KY 40508
- University of Kentucky College of Pharmacy, Lexington, KY 40508
- Markey Cancer Center, Lexington, KY 40508
Corresponding Author
Michael Cavnar, MD
Department of Surgery
University of Kentucky
800 Rose Street, C220
Lexington, KY 40508
Phone: (859) 323-8920
Email: michael.cavnar@uky.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 27, 2020
Revision received: October 6, 2020
Accepted: November 1, 2020