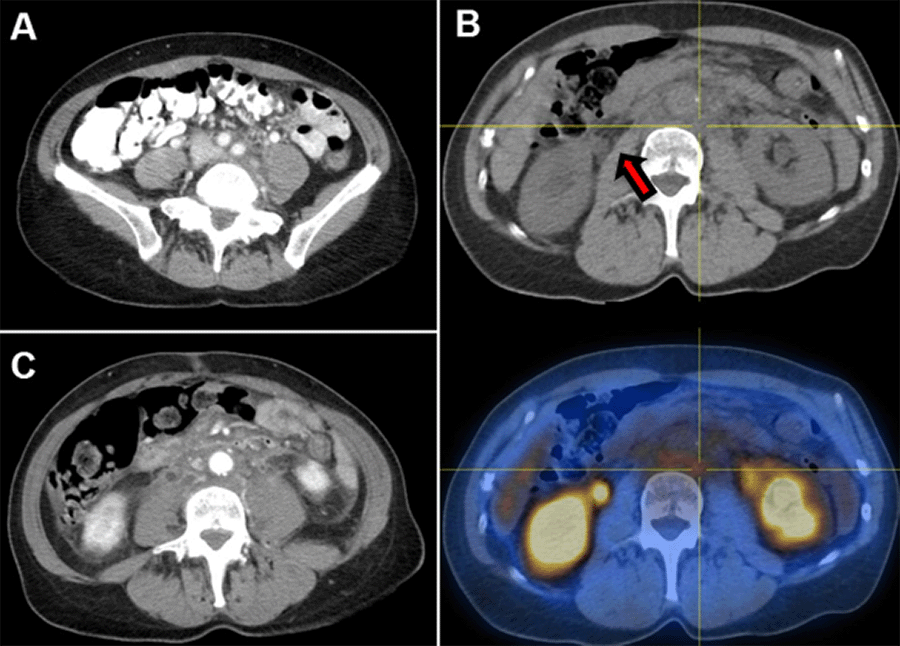
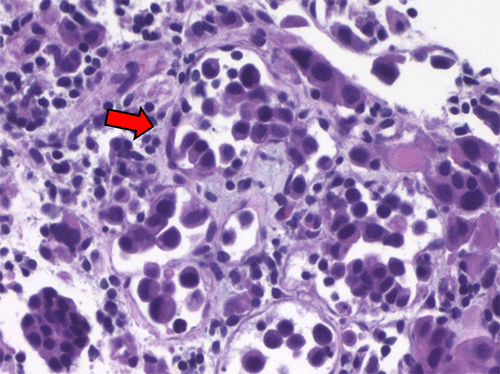
Discussion
Gastric cancer is the sixth most common malignancy worldwide, with higher incidence and prevalence rates in Asia. It has the fifth-highest mortality rate globally, though this is much lower in the United States, where there is a reduced incidence and prevalence.1‒4 Gastric cancer has an indolent course, detected after patients become symptomatic, largely from mass effect of the primary mass, paraneoplastic syndromes, or metastatic burden. For this reason, approximately 50% of patients, at the time of presentation, have disease that is already beyond locoregional confines and is unresectable.5 Adenocarcinoma represents 95% of gastric cancers and can be divided into intestinal or diffuse types based on the Lauren classification or into papillary, tubular, mucinous, and poorly cohesive types based on the World Health Organization (WHO) classification in 2010. The patient described above falls into the diffuse or poorly cohesive type, which occurs equally in men and women, is more common in people <50 years of age, and is associated with hereditary diffuse gastric cancer. It is marked by flat or ulcerated growth patterns, linitis plastica, and loss of cohesion with signet ring cells and no glands.6
Retroperitoneal fibrosis (RPF) is an uncommon disease process characterized by the presence of inflammatory and fibrous tissue around the infrarenal abdominal aorta and iliac arteries, often encasing the ureters.7 RPF has an annual incidence of 1.3 per 100,000 persons per year. The differential diagnosis for RPF is broad, including not only iatrogenic causes such as radiation, abdominal or pelvic surgeries, but also trauma, infection, and inflammatory states; however, 70% of cases are idiopathic, and 8% are attributed to malignancies, most commonly carcinoid, which has been associated with serotonin release, and Hodgkin and non-Hodgkin lymphomas, sarcomas, colorectal, bladder, prostate, and breast cancers, which have been associated with a desmoplastic reaction.8‒10 Like gastric adenocarcinoma, RPF (thought to be due to the aforementioned desmoplastic reaction) is generally a delayed diagnosis, often only detected after causing organ dysfunction, most commonly ureteral obstruction or renal impairment. This etiology of ureteral obstruction is distinct from that caused by external compression from pelvic peritoneal disease due to gastric cancer. Other common presentations of retroperitoneal fibrosis are nonspecific, including lower back and flank pain, malaise, anorexia, weight loss, fevers, nausea, and vomiting.7 Gastric cancers can present with additional nonspecific symptoms such as abdominal pain, anorexia, nausea, early satiety, and weight loss as well as dysphagia, hematemesis, and dyspepsia.11
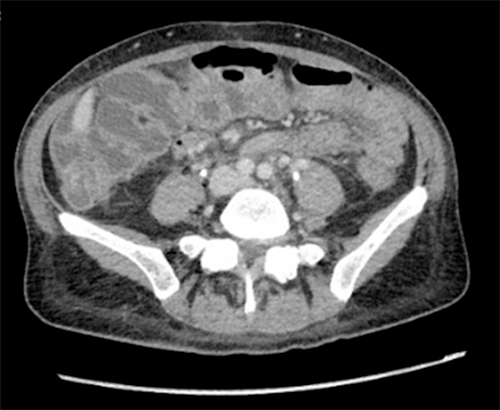
Globally, there have been few reported cases of RPF leading to the diagnosis of gastric cancer, two such cases in Japan and one in Iran; all reported cases have been associated with scirrhous gastric adenocarcinoma.12‒14 Only a single case report from the United States has been published describing a patient with gastric adenocarcinoma and concurrent RPF. Peixoto et al. presented a patient with renal failure and jaundice secondary to encasement and mass effect of RPF; it was only during attempted EUS of the RPF mass that she was found to have a thickened gastric wall. Gastric biopsy demonstrated gastric adenocarcinoma with linitis plastica. This patient could not undergo palliative chemotherapy due to reduced performance status and organ dysfunction. Because poor performance status is a common feature of patients with cancer and metastatic RPF, the effect of chemotherapy on malignancy-associated RPF is unknown.15 Our patient is unique in that she could initiate chemotherapy immediately after staging laparoscopy since the optimization of renal function was achieved with ureteral stents. After completing three cycles of FOLFOX with Herceptin, she was readmitted to the hospital for unrelated causes. At that time, a CT scan was completed (Figure 4), revealing interval improvement of RPF.
Figure 4. Retroperitoneal Fibrosis Associated With Gastric Cancer Improved After Three Cycles of Chemotherapy. Published With Permission
Axial image from CT abdomen/pelvis with IV contrast illustrating interval decrease in RPF at level of aortic bifurcation following three cycles of FOLFOX/Herceptin.
Conclusion
Rarely associated with gastric adenocarcinoma, RPF detection warrants complete and expedient workup. If the functional status of these patients allows them to receive palliative chemotherapy, they may have a reduced burden of RPF disease.
Lessons Learned
Retroperitoneal fibrosis is an uncommon disease process, infrequently associated with metastatic cancer. The effect of chemotherapy on RPF disease burden in patients with gastric adenocarcinoma is poorly understood due to the delayed presentation of patients with advanced organ failure and reduced functional status. Timely diagnosis of malignancy-associated RPF may allow patients to receive palliative chemotherapy to delay RPF progression and improve quality of life.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin. 2020 Jul;70(4):313]. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. doi:10.1002/ijc.31937
- Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27. doi:10.1158/1055-9965.EPI-15-0578
- de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42(2):219-240. doi:10.1016/j.gtc.2013.01.003
- Mansfield PF. Clinical features, diagnosis, and staging of gastric cancer. UpToDate. 2020.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi:10.1002/ijc.25516
- Vaglio A, Palmisano A. Clinical manifestations and diagnosis of retroperitoneal fibrosis. UpToDate. 2020.
- van Bommel EFH, Jansen I, Hendriksz TR, Aarnoudse ALHJ. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore). 2009;88(4):193-201. doi:10.1097/MD.0b013e3181afc420
- Koep L, Zuidema GD. The clinical significance of retroperitoneal fibrosis. Surgery. 1977;81(3):250-257.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002;60(6):231-242.
- Recio-Boiles A, Babiker HM. Gastric Cancer. [Updated 2021 Dec 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Yokoyama R, Tazaki R, Morita H, et al. Retroperitoneal fibrosis in a patient with gastric cancer manifested by lower extremity edema and hydrocele. Intern Med. 2012;51(16):2157-2160. doi:10.2169/internalmedicine.51.7660
- Dohmen K, Mizukami Y, Tanaka K, et al. Retroperitoneal fibrosis associated with scirrhous gastric cancer. Gastroenterol Jpn. 1993;28(5):699-705. doi:10.1007/BF02806351
- Karbasi A, Karbasi-Afshar R, Ahmadi J, Saburi A. Retroperitoneal fibrosis as a result of signet ring cell gastric cancer: a case-based review. J Gastrointest Cancer. 2013;44(1):94-97. doi:10.1007/s12029-012-9422-1
- Peixoto RD, Al-Barrak J, Lim H, Renouf D. Gastroesophageal cancer and retroperitoneal fibrosis: Two case reports and review of the literature. World J Gastrointest Oncol. 2013;5(3):68-70. doi:10.4251/wjgo.v5.i3.68
Authors
Podrat JLa; Chegireddy Va; Sheu Tb; Anton Rb; Satkunasivam Rc; Holder AMa
Author Affiliations
- Department of Surgery, Houston Methodist Hospital, Houston, TX 77030
- Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX 77030
- Department of Urology, Houston Methodist Hospital, Houston, TX 77030
Corresponding Author
Ashley M. Holder, MD, FACS
Division of Surgical Oncology
University of Alabama Birmingham
1808 7th Avenue South, BDB 571
Birmingham, AL 35294
Email: amholder@uab.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 18, 2020
Revision received: November 2, 2020
Accepted: December 7, 2020