Discussion
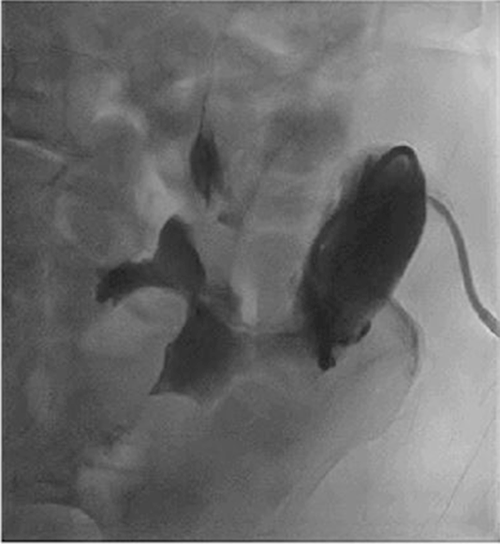
Marginal ulcers, previously a common complication after procedures involving upper GI reconstruction, are now less prevalent with the advent of proton pump inhibitors.1 In the setting of PD, a review of the literature revealed that the occurrence of marginal ulcers ranges from 1 to 9.4%.2‒4 In a study by Sakaguchi et al. that reported 72 patients who underwent standard PD and 28 who underwent PPPD,4 14.3% patients developed marginal ulceration after PPPD compared to 0 (0%) patients who underwent standard PD. Of the four patients with marginal ulcers, three patients were reconstructed with the Roux-en-Y method, and one patient was reconstructed via a pancreaticogastrostomy. In the Roux-en-Y method, the distal jejunal loop anastomosed to the bulb was directly exposed to gastric juice without neutralization by pancreatic juice from the proximal jejunal limb, increasing the risk for marginal ulcers.5 In our patient, our PPPD reconstruction was done in a Billroth II fashion allowing pancreatic juices to neutralize acidic gastric juices from the stomach (Figure 5). Patients who undergo PPPD at our institution are also discharged with a PPI.
Figure 5. Types of Reconstruction Following PPPD. Published With Permission
PD has been increasing in recent years as a safe and appropriate option for resection in selected patients with malignant and benign disorders of the pancreas and periampullary region. Common postoperative complications include delayed gastric emptying, disruption of the pancreatic-enteric anastomosis with subsequent pancreatic fistula, wound infection, and hemorrhage.4
The incidence of marginal ulcers after PD ranges from as low as 1% up to 9.4%.2‒4 Marginal ulcers often present with vague abdominal symptoms such as pain, dysphagia, nausea, and vomiting. These symptoms are usually managed conservatively with a six-week course consisting of acid-blocking agents and cytoprotective agents such as sucralfate. NSAIDs should also be discontinued, and patients should be encouraged to stop smoking. Although medical management of marginal ulcers is successful in 85 to 95% of patients, surgery—revision of the gastro or duodenojejunostomy with resection and vagotomy (in stable patients)—or Graham patch, abdominal washout, feeding tube placement (in unstable patients) may be indicated if marginal ulcers perforate or if persistent pain or recurrent bleeding occurs despite maximal medical therapy.6‒9
With the rapid evolution of interventional endoscopic techniques in recent years, less invasive options are now available to manage a broad range of GI pathology. They can also be utilized to manage postoperative complications. These innovative interventional endoscopic techniques have included the development of endoscopic closure techniques such as clipping, stenting, suturing, gluing, and endoscopic vacuum therapy and revolutionized the management of GI defects. Interventional endoscopic techniques typically provide a more affordable alternative to surgery with less morbidity and resource utilization.10
Fistulas in the GI tract are epithelialized tracts that are continuously exposed to GI secretions, whereby the resulting inflammation poses significant challenges in closure. These fistulas are frequently acid-related, related to a failure of healing, malignant, or have associated radiation injury, which alters the anatomy and causes fibrotic changes, further complicating the management.
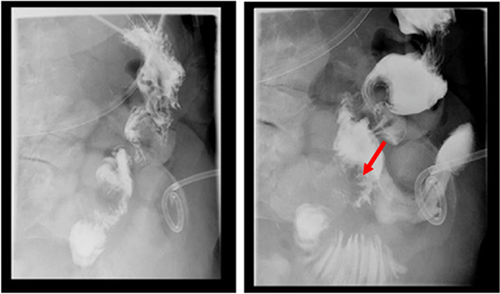
Gastric fistulas, though rare, can be seen after bariatric surgery, percutaneous endoscopic gastrostomy tube removal, or as in our case, may result from a marginal ulcer.11 In these settings, endoscopic intervention ranges from clips to adhesive glue application, endoscopic suturing, to stenting. Endoscopic clips, mostly the over-the-scope clip (OTSC), have been the most successful (80.3%) in the immediate closure of esophageal, gastric and colonic fistulas. However, long-term success has not been tested or is disappointing, with a high fistula recurrence rate (19.7%).12 Less studied, although seemingly promising, endoscopic suture closure of fistulas (mean fistula size=9 mm) had 100% and 80% initial and long-term clinical success in patients with GI fistulas, respectively (n=40). Long-term clinical success was more likely if the fistula was closed within 30 days of diagnosis as compared with >30 days after diagnosis (69% vs. 23%, respectively; P=0.037). Though this study suggests that endoscopic suturing can be used to close larger fistulas with short-term success, its long-term clinical efficacy is limited by the lack of data and the high recurrence in available retrospective studies.13
Endoscopic devices and technical innovation promise new and less invasive techniques to manage a wide range of GI disruptions. With trends leaning towards endoscopic management of GI leaks, perforations, and fistulas and studies proving good patient outcomes, management of these GI disruptions involves a multidisciplinary team consisting of advanced endoscopists, surgeons, and interventional radiology, as appropriate.11 While endoscopic closure can be highly successful in cases identified early and with minimal extra-luminal contamination, surgical or interventional radiology-directed drainage may be warranted in cases of uncontained perforation, delayed recognition, or gross contamination. Therefore, the decision regarding conservative, endoscopic, or surgical management should be individualized.
Conclusion
In the setting of gastrointestinal operations, gastrointestinal disruptions are well-described complications. We present a case of a marginal ulcer presenting 18 months after a PPPD as a fistula that was managed with interventional radiology drainage, endoscopic suturing, and stenting. We conclude that management of GI disruptions after GI procedures should involve a multidisciplinary team to individualize treatment options and optimize patient outcomes.
Lessons Learned
Endoscopic devices and technical innovation promise new and less invasive management options for a wide range of GI disruptions. While endoscopic closure proves to be highly successful in cases identified early and with minimal extra-luminal contamination, surgical repair or interventional radiology-directed drainage may be warranted in cases of uncontained perforation, delayed recognition, or gross contamination. Therefore, conservative, radiographic, endoscopic, or surgical management should be individualized to the patient's needs.
References
- Ying VW, Kim SH, Khan KJ, et al. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: a systematic review and meta-analysis of cohort studies. Surg Endosc. 2015;29(5):1018-1023. doi:10.1007/s00464-014-3794-1
- Wu JM, Tsai MK, Hu RH, Chang KJ, Lee PH, Tien YW. Reflux esophagitis and marginal ulcer after pancreaticoduodenectomy. J Gastrointest Surg. 2011;15(5):824-828. doi:10.1007/s11605-011-1463-4
- Grace PA, Pitt HA, Longmire WP. Pylorus preserving pancreatoduodenectomy: an overview. Br J Surg. 1990;77(9):968-974. doi:10.1002/bjs.1800770906
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248-260. doi:10.1097/00000658-199709000-00004
- Sakaguchi T, Nakamura S, Suzuki S, et al. Marginal ulceration after pylorus-preserving pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2000;7(2):193-197. doi:10.1007/s005340050175
- Sanyal AJ, Sugerman HJ, Kellum JM, Engle KM, Wolfe L. Stomal complications of gastric bypass: incidence and outcome of therapy. Am J Gastroenterol. 1992;87(9):1165-1169.
- Dallal RM, Bailey LA. Ulcer disease after gastric bypass surgery. Surg Obes Relat Dis. 2006;2(4):455-459. doi:10.1016/j.soard.2006.03.004
- Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients--what have we learned?. Obes Surg. 2000;10(6):509-513. doi:10.1381/096089200321593706
- Moon RC, Teixeira AF, Goldbach M, Jawad MA. Management and treatment outcomes of marginal ulcers after Roux-en-Y gastric bypass at a single high volume bariatric center. Surg Obes Relat Dis. 2014;10(2):229-234. doi:10.1016/j.soard.2013.10.002
- Singh RR, Nussbaum JS, Kumta NA. Endoscopic management of perforations, leaks and fistulas. Transl Gastroenterol Hepatol. 2018;3:85. Published 2018 Oct 31. doi:10.21037/tgh.2018.10.09
- Falconi M, Pederzoli P. The relevance of gastrointestinal fistulae in clinical practice: a review. Gut. 2001;49 Suppl 4(Suppl 4):iv2-iv10. doi:10.1136/gut.49.suppl_4.iv2
- Manta R, Caruso A, Cellini C, et al. Endoscopic management of patients with post-surgical leaks involving the gastrointestinal tract: A large case series. United European Gastroenterol J. 2016;4(6):770-777. doi:10.1177/2050640615626051
- Sharaiha RZ, Kumta NA, DeFilippis EM, et al. A large multicenter experience with endoscopic suturing for management of gastrointestinal defects and stent anchorage in 122 patients: a retrospective review. J Clin Gastroenterol. 2016;50(5):388-392. doi:10.1097/MCG.0000000000000336
Authors
Tham Ea; Hewitt DBa; Bakhit MMb; Kowalski TEb; Yeo CJa
Author Affiliations
- Department of Surgery, Thomas Jefferson University Hospital, Philadelphia, PA 19107
- Division of Gastroenterology and Hepatology, Department of Medicine, Jefferson Digestive Health Institute, Philadelphia, PA 19107
Corresponding Author
Elwin Tham, MD
Department of Surgery
Thomas Jefferson University
1015 Walnut Street
Curtis Building, Ste. 620
Philadelphia, PA 19107
Phone: (215) 694-9281
Email: ext036@jefferson.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 27, 2020
Accepted: October 5, 2020