Discussion
Intraoperative localization of gastrointestinal tract lesions identified by upper or lower endoscopy can be challenging, especially in the era of minimally invasive surgery where there is no haptic feedback to assist in identification. Preoperative marking was selected to eliminate the need for intraoperative endoscopy or anterior gastrostomy. Consequently, many preoperative marking techniques have been described, such as endoscopic clipping,1 computer-assisted endoscopic marking,2 and indocyanine green injection with intraoperative fluoroscopic guidance. However, the most frequently performed and well-studied technique is endoscopic tattooing, achieved by injection of submucosal dye.3 Since it was first reported by Sauntry et al. in 1958, endoscopic tattooing for gastrointestinal lesion localization has been widely used worldwide.4 Endoscopic tattooing has been described for preoperative marking of esophageal, gastric, pancreatic, small intestinal, and colorectal lesions.3 Other reported benefits of endoscopic tattooing include an increased number of harvested lymph nodes in colon cancer and improved identification of primary tumors following neoadjuvant chemoradiation in locally advanced rectal cancer.5
Many researchers studied the safety and efficacy of different dyes in endoscopic tattooing. Hammond et al.6 assessed eight tattooing agents in an animal model and demonstrated that only indocyanine green and India ink (diluted carbon particles) could persist beyond two days. Still, one drawback of India ink was severe inflammatory reactions. Recently, SPOT (GI Supply, Camp Hill, Pennsylvania, USA), consisting of sterile purified carbon particles, has been FDA-approved as a tattooing agent.3
Technically, optimal endoscopic tattooing is achieved with precise submucosal injection of dye at a critical depth to provide extraluminal visibility of the tattoo for ease of intraoperative detection. However, known risks of this technique include transmural injections that can cause perforation or tumor inoculation. Therefore, two methods have been described for consistent injections within the submucosa only: 1) creation of a saline bleb to facilitate dye injection or 2) direct injection of the dye into the submucosa by approaching the mucosa tangentially and lifting the needle toward the center of the lumen with a controlled, slow injection after creating a small submucosal bleb.7
According to many case series, the reported risk of clinical complications after endoscopic tattooing is relatively low (0.22-5.6%).3,5,8,9 Nizam et al. published a large review of 447 cases, with a reported risk of clinical complications of 0.22%; furthermore, most of these complications were related to transmural injection.8 Other case reports note minor complications, including dye spillage (2.4%), focal peritonitis, infected hematoma, and abscess formation.5 Of these complications, tumor inoculation is one of the risks that can lead to tumor spread or locoregional recurrence. However, all case reports are in preoperative tattooing of patients with colorectal cancers, not upper gastrointestinal malignancies.10‒12 A single case of gastric laceration and hematemesis associated with endoscopic tattooing has been published.13 Furthermore, other studies describe the association of more invasive upper gastrointestinal endoscopic procedures, such as percutaneous endoscopic gastrostomy, endoscopic submucosal dissection, and endoscopic mucosal resection, with a risk of gastric perforation14,15 and peritoneal seeding.16,17
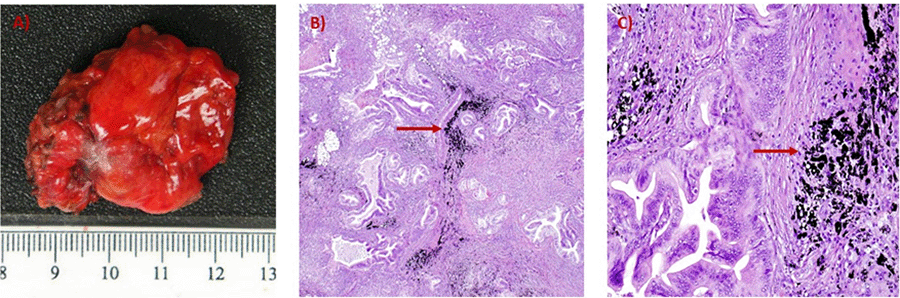
Our patient is unique as she represents the first report of the presence of tattoo pigment in a peritoneal metastasis and lymph nodes two years following total gastrectomy and D2 lymphadenectomy in early-stage gastric cancer. Our case highlights the potential risk of endoscopic tattooing in patients with upper gastrointestinal cancers and the importance of imaging surveillance to identify locoregional or distant recurrences.
Conclusion
Endoscopic tattooing is potentially risky for peritoneal seeding and locoregional recurrence, even in early-stage cancers.
Lessons Learned
Endoscopic tattooing is used to facilitate intraoperative identification of gastrointestinal lesions. However, in patients at risk for or with known invasive cancer, alternative localizing techniques, such as intraoperative endoscopy, should be considered.
References
- Matsuda T, Iwasaki T, Hirata K, et al. Simple and reliable method for tumor localization during totally laparoscopic gastrectomy: intraoperative laparoscopic ultrasonography combined with tattooing. Gastric Cancer. 2017;20(3):548-552. doi:10.1007/s10120-016-0635-z
- Hu W, Wang B, Sun L, et al. Computer-simulated biopsy marking system for endoscopic surveillance of gastric lesions: a pilot study. Biomed Res Int. 2015;2015:197270. doi:10.1155/2015/197270
- Luigiano C, Ferrara F, Morace C, et al. Endoscopic tattooing of gastrointestinal and pancreatic lesions. Adv Ther. 2012;29(10):864-873. doi:10.1007/s12325-012-0056-2
- Sauntry JP, Knudtson KP. A technique for marking the mucosa of the gastrointestinal tract after polypectomy. Cancer. 1958;11(3):607-610. doi:10.1002/1097-0142(195805/06)11:3<607::aid-cncr2820110322>3.0.co;2-y
- Trakarnsanga A, Akaraviputh T. Endoscopic tattooing of colorectal lesions: Is it a risk-free procedure?. World J Gastrointest Endosc. 2011;3(12):256-260. doi:10.4253/wjge.v3.i12.256
- Hammond DC, Lane FR, Welk RA, Madura MJ, Borreson DK, Passinault WJ. Endoscopic tattooing of the colon. An experimental study. Am Surg. 1989;55(7):457-461.
- Rex DK. The Appropriate Use and Techniques of Tattooing in the Colon. Gastroenterol Hepatol (N Y). 2018;14(5):314-317.
- Nizam R, Siddiqi N, Landas SK, Kaplan DS, Holtzapple PG. Colonic tattooing with India ink: benefits, risks, and alternatives. Am J Gastroenterol. 1996;91(9):1804-1808.
- Ponsky JL, King JF. Endoscopic marking of colonic lesions. Gastrointest Endosc. 1975;22(1):42-43. doi:10.1016/s0016-5107(75)73687-8
- Uehara H, Yamazaki T, Iwaya A, et al. A rare case of peritoneal deposits with carbon pigmentation after preoperative endoscopic tattooing for sigmoid colon cancer. Int J Colorectal Dis. 2019;34(2):355-358. doi:10.1007/s00384-018-3189-1
- Sun B. Endoscopic tattooing: a risk for tumor implantation. Int J Colorectal Dis. 2020;35(3):571-574. doi:10.1007/s00384-019-03495-9
- Cappell MS, Courtney JT, Amin M. Black macular patches on parietal peritoneum and other extraintestinal sites from intraperitoneal spillage and spread of India ink from preoperative endoscopic tattooing: an endoscopic, surgical, gross pathologic, and microscopic study. Dig Dis Sci. 2010;55(9):2599-2605. doi:10.1007/s10620-009-1044-5
- Gonzalez-Tallon AI, Rivero-Fernandez M, Calvo-Ramos I, et al. Hematemesis With Gastric Laceration After Tattooing a Polyp With Purified Carbon: A Review of the Literature. Gastroenterology Res. 2017;10(1):45-49. doi:10.14740/gr728w
- Huh CW, Kim GJ, Kim BW, Seo M, Kim JS. Long-term Clinical Outcomes and Risk of Peritoneal Seeding after Endoscopic Submucosal Dissection for Early Gastric Cancer: A Focus on Perforation during the Procedure. Gut Liver. 2019;13(5):515-521. doi:10.5009/gnl18350
- Ikehara H, Gotoda T, Ono H, Oda I, Saito D. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94(8):992-995. doi:10.1002/bjs.5636
- Hirao M, Yamada T, Michida T, et al. Peritoneal Seeding after Gastric Perforation during Endoscopic Submucosal Dissection for Gastric Cancer. Dig Surg. 2018;35(5):457-460. doi:10.1159/000481715
- Gleeson FC, Lee JH, Dewitt JM. Tumor Seeding Associated With Selected Gastrointestinal Endoscopic Interventions. Clin Gastroenterol Hepatol. 2018;16(9):1385-1388. doi:10.1016/j.cgh.2018.05.014
Authors
Soliman BGa; Lim Sa; Schwartz MRb; Krishnan Kc; Bernicker EHd; Holder AMa
Author Affiliations
- Department of Surgery, Houston Methodist Hospital, Houston, TX 77030
- Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX 77030
- Department of Medicine, Massachusetts General Hospital, Boston, MA 02114
- Department of Medical Oncology, Houston Methodist Cancer Center, Houston, TX 77030
Corresponding Author
Ashley M. Holder, FACS
Division of Surgical Oncology
University of Alabama at Birmingham
1808 7th Avenue South, BDB 571
Birmingham, AL 35294
Email: amholder@uab.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 18, 2020
Revision received: September 29, 2020
Accepted: December 7, 2020